Structure ( IF 5.7 ) Pub Date : 2023-03-28 , DOI: 10.1016/j.str.2023.03.004 Jiuyang Liu 1 , Belén Chaves-Arquero 2 , Pengcheng Wei 3 , Adam H Tencer 1 , Antonio Ruiz-Albor 2 , Gongyi Zhang 3 , Francisco J Blanco 2 , Tatiana G Kutateladze 1
|
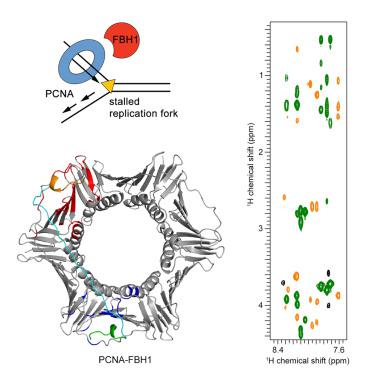
F-box DNA helicase 1 (FBH1) is involved in the regulation of cell responses to replicative stress. FBH1 is recruited to stalled DNA replication fork by PCNA where it inhibits homologous recombination and catalyzes fork regression. Here, we report the structural basis for the molecular recognition of two distinctly different motifs of FBH1, FBH1PIP and FBH1APIM, by PCNA. The crystal structure of PCNA in complex with FBH1PIP and analysis of NMR perturbations reveal overlapped FBH1PIP and FBH1APIM binding sites of PCNA and the dominant contribution of FBH1PIP in this interaction.
中文翻译:

FBH1 PCNA 结合模式的分子洞察
F-box DNA 解旋酶 1 (FBH1) 参与调节细胞对复制应激的反应。FBH1 被 PCNA 招募到停滞的 DNA 复制叉,在那里它抑制同源重组并催化叉回归。在这里,我们报告了PCNA 对PIP和 FBH1APIM两种截然不同的基序进行分子识别的结构基础。与 FBH1 PIP复合的 PCNA 的晶体结构和 NMR 扰动分析揭示了PCNAPIP和 FBH1APIMPIP在这种相互作用中的主要贡献。



























 京公网安备 11010802027423号
京公网安备 11010802027423号