Journal of Hepatology ( IF 26.8 ) Pub Date : 2023-03-28 , DOI: 10.1016/j.jhep.2023.03.016 Feng He 1 , Peng Zhang 2 , Junlai Liu 2 , Ruolei Wang 3 , Randal J Kaufman 4 , Benjamin C Yaden 5 , Michael Karin 6
|
Background & Aims
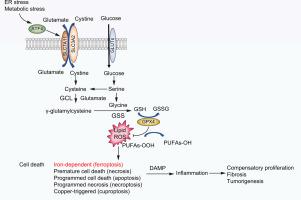
Hepatocellular carcinoma (HCC), a leading cause of cancer-related death, is associated with viral hepatitis, non-alcoholic steatohepatitis (NASH), and alcohol-related steatohepatitis, all of which trigger endoplasmic reticulum (ER) stress, hepatocyte death, inflammation, and compensatory proliferation. Using ER stress-prone MUP-uPA mice, we established that ER stress and hypernutrition cooperate to cause NASH and HCC, but the contribution of individual stress effectors, such as activating transcription factor 4 (ATF4), to HCC and their underlying mechanisms of action remained unknown.
Methods
Hepatocyte-specific ATF4-deficient MUP-uPA mice (MUP-uPA/Atf4Δhep) and control MUP-uPA/Atf4F/F mice were fed a high-fat diet to induce NASH-related HCC, and Atf4F/F and Atf4Δhep mice were injected with diethylnitrosamine to model carcinogen-induced HCC. Histological, biochemical, and RNA-sequencing analyses were performed to identify and define the role of ATF4-induced solute carrier family 7a member 11 (SLC7A11) expression in hepatocarcinogenesis. Reconstitution of SLC7A11 in ATF4-deficient primary hepatocytes and mouse livers was used to study its effects on ferroptosis and HCC development.
Results
Hepatocyte ATF4 ablation inhibited hepatic steatosis, but increased susceptibility to ferroptosis, resulting in accelerated HCC development. Although ATF4 activates numerous genes, ferroptosis susceptibility and hepatocarcinogenesis were reversed by ectopic expression of a single ATF4 target, Slc7a11, coding for a subunit of the cystine/glutamate antiporter xCT, which is needed for glutathione synthesis. A ferroptosis inhibitor also reduced liver damage and inflammation. ATF4 and SLC7A11 amounts were positively correlated in human HCC and livers of patients with NASH.
Conclusions
Despite ATF4 being upregulated in established HCC, it serves an important protective function in normal hepatocytes. By maintaining glutathione production, ATF4 inhibits ferroptosis-dependent inflammatory cell death, which is known to promote compensatory proliferation and hepatocarcinogenesis. Ferroptosis inhibitors or ATF4 activators may also blunt HCC onset.
Impact and implications
Liver cancer or hepatocellular carcinoma (HCC) is associated with multiple aetiologies. Most HCC aetiologies cause hepatocyte stress and death, as well as subsequent inflammation, and compensatory proliferation, thereby accelerating HCCdevelopment. The contribution of individual stress effectors to HCC and their underlying mechanisms of action were heretofore unknown. This study shows that the stress-responsive transcription factor ATF4 blunts liver damage and cancer development by suppressing iron-dependent cell death (ferroptosis). Although ATF4 ablation prevents hepatic steatosis, it also increases susceptibility to ferroptosis, due to decreased expression of the cystine/glutamate antiporter SLC7A11, whose expression in human HCC and NASH correlates with ATF4. These findings reinforce the notion that benign steatosis may be protective and does not increase cancer risk unless accompanied by stress-induced liver damage. These results have important implications for prevention of liver damage and cancer.
中文翻译:

ATF4 通过诱导 SLC7A11 (xCT) 阻断应激相关的铁死亡来抑制肝癌发生
背景与目标
肝细胞癌 (HCC) 是癌症相关死亡的主要原因,与病毒性肝炎、非酒精性脂肪性肝炎 (NASH) 和酒精相关性脂肪性肝炎有关,所有这些都会引发内质网 (ER) 应激、肝细胞死亡、炎症和补偿性增殖。使用易发生内质网应激的MUP-uPA小鼠,我们确定内质网应激和营养过剩共同导致 NASH 和 HCC,但个体应激效应物(例如激活转录因子 4 (ATF4))对 HCC 的贡献及其潜在作用机制仍然未知。
方法
肝细胞特异性 ATF4 缺陷型MUP-uPA小鼠 ( MUP-uPA/Atf4 Δhep ) 和对照MUP-uPA/Atf4 F/F小鼠喂食高脂饮食以诱导 NASH 相关 HCC, Atf4 F/F和Atf4 Δhep小鼠注射二乙基亚硝胺来模拟致癌物诱导的 HCC。通过组织学、生化和 RNA 测序分析来鉴定和定义 ATF4 诱导的溶质载体家族 7a 成员 11 (SLC7A11) 表达在肝癌发生中的作用。在 ATF4 缺陷的原代肝细胞和小鼠肝脏中重建 SLC7A11 用于研究其对铁死亡和 HCC 发展的影响。
结果
肝细胞 ATF4 消融抑制了肝脂肪变性,但增加了铁死亡的易感性,导致 HCC 加速发展。尽管 ATF4 激活许多基因,但单个 ATF4 靶标 Slc7a11 的异位表达可逆转铁死亡易感性和肝癌发生, Slc7a11编码胱氨酸/谷氨酸逆向转运蛋白 xCT 的亚基,这是谷胱甘肽合成所需的。铁死亡抑制剂还可以减少肝脏损伤和炎症。 ATF4和SLC7A11 的量在人类 HCC 和 NASH 患者的肝脏中呈正相关。
结论
尽管 ATF4 在已形成的 HCC 中表达上调,但它在正常肝细胞中发挥着重要的保护功能。通过维持谷胱甘肽的产生,ATF4 抑制铁死亡依赖性炎症细胞死亡,众所周知,炎症细胞死亡会促进代偿性增殖和肝癌发生。铁死亡抑制剂或 ATF4 激活剂也可能减缓 HCC 的发病。
影响和影响
肝癌或肝细胞癌 (HCC) 与多种病因相关。大多数 HCC 病因会导致肝细胞应激和死亡,以及随后的炎症和代偿性增殖,从而加速 HCC 的发展。迄今为止,个体应激效应因子对 HCC 的贡献及其潜在作用机制尚不清楚。这项研究表明,应激反应转录因子 ATF4 通过抑制铁依赖性细胞死亡(铁死亡)来减轻肝损伤和癌症发展。虽然 ATF4 消除可以预防肝脂肪变性,但由于胱氨酸/谷氨酸逆向转运蛋白 SLC7A11 的表达减少,它也会增加铁死亡的易感性,而该蛋白在人类 HCC 和 NASH 中的表达与 ATF4 相关。这些发现强化了这样的观念:良性脂肪变性可能具有保护作用,并且不会增加癌症风险,除非伴有压力引起的肝损伤。这些结果对于预防肝损伤和癌症具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号