Structure ( IF 4.4 ) Pub Date : 2023-03-23 , DOI: 10.1016/j.str.2023.02.013 Sudarshan Tandukar 1 , Eunju Kwon 1 , Dong Young Kim 1
|
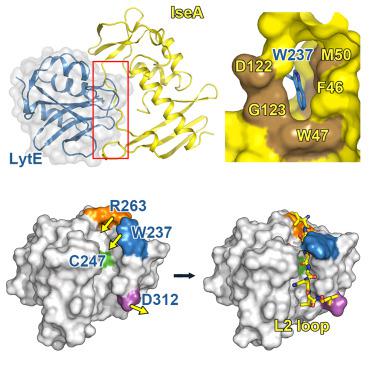
Peptidoglycan, a physical barrier that protects bacteria from the environment, is constantly degraded and resynthesized for remodeling during cell growth and division. Because excessive or insufficient peptidoglycan hydrolysis affects bacterial homeostasis and viability, peptidoglycan degradation must be precisely regulated. In Bacillus subtilis, DL-endopeptidases play an essential role in peptidoglycan remodeling, and their activity is regulated by IseA. Here, we report the crystal structure of peptidoglycan DL-endopeptidase LytE complexed with IseA. In the crystal structure, the inhibitory loop connecting the two lobes of IseA blocks the active site of LytE by mimicking its substrate. Consistently, mutations in the inhibitory loop resulted in the loss of IseA activity. The structure also shows that conformational rearrangements in both LytE and IseA restrict access of the inhibitory loop to the LytE catalytic site. These results reveal an inhibition mechanism of peptidoglycan DL-endopeptidase in which the inhibitory protein mimics the substrate but is not degraded.
中文翻译:

抑制蛋白 IseA 对肽聚糖 DL-内肽酶调控的结构洞察
肽聚糖是一种保护细菌免受环境影响的物理屏障,在细胞生长和分裂过程中不断降解和重新合成以进行重塑。由于过多或不足的肽聚糖水解会影响细菌稳态和生存能力,因此必须精确调节肽聚糖降解。在枯草芽孢杆菌中, DL-肽链内切酶在肽聚糖重塑中起重要作用,其活性受 IseA 调节。在这里,我们报告了与 IseA 复合的肽聚糖 DL-内肽酶 LytE 的晶体结构。在晶体结构中,连接 IseA 两个裂片的抑制环通过模仿 LytE 的底物来阻断 LytE 的活性位点。一致地,抑制环中的突变导致 IseA 活性丧失。该结构还表明,LytE 和 IseA 中的构象重排限制了抑制环进入 LytE 催化位点。这些结果揭示了肽聚糖 DL-内肽酶的抑制机制,其中抑制蛋白模拟底物但未降解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号