当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alternative Approach to the Large-Scale Synthesis of the Densely Functionalized Pyrrolidone BMT-415200
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-03-22 , DOI: 10.1021/acs.oprd.3c00007 Souvik Rakshit 1 , Shunmugaraj Sathasivam 1 , Raghurami Reddy 1 , Saladi V. Rao 1 , Vijaykumar Shekarappa 1 , Moorthy Kandasamy 1 , Mohamed Jaleel 1 , Saravanan Murugan 1 , Anuradha Bhat 1 , Tamilarasan Subramani 1 , Thirumalai Lakshminarasimhan 1 , Aravind Gangu 1 , Steven R. Wisniewski 2 , Nathaniel Kopp 2 , Ian Hale 2 , Jason M. Stevens 2 , Victor W. Rosso 2 , Martin D. Eastgate 2 , Rajappa Vaidyanathan 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-03-22 , DOI: 10.1021/acs.oprd.3c00007 Souvik Rakshit 1 , Shunmugaraj Sathasivam 1 , Raghurami Reddy 1 , Saladi V. Rao 1 , Vijaykumar Shekarappa 1 , Moorthy Kandasamy 1 , Mohamed Jaleel 1 , Saravanan Murugan 1 , Anuradha Bhat 1 , Tamilarasan Subramani 1 , Thirumalai Lakshminarasimhan 1 , Aravind Gangu 1 , Steven R. Wisniewski 2 , Nathaniel Kopp 2 , Ian Hale 2 , Jason M. Stevens 2 , Victor W. Rosso 2 , Martin D. Eastgate 2 , Rajappa Vaidyanathan 1
Affiliation
|
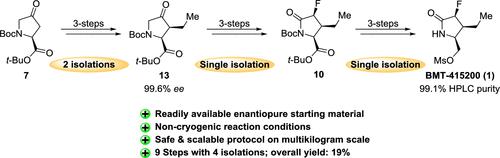
The development of a multi-kilogram-scale synthetic route to enantiomerically pure ((2S,3S,4S)-3-ethyl-4-fluoro-5-oxopyrrolidin-2-yl)methyl methanesulfonate (BMT-415200) 1 is described in this work. In this sequence, a safe and robust process of nine linear steps with four isolations was implemented. The synthesis features highly diastereoselective hydrogenation of enones 12, diastereoselective reduction of ketone 13, and deoxyfluorination of the corresponding secondary alcohol 8 followed by C–H oxidation of 9 to lactam 10. The target compound 1 was prepared in 19% overall yield with >99% purity from commercially available di-tert-butyl (S)-4-oxopyrrolidine-1,2-dicarboxylate 7.
中文翻译:

大规模合成密集功能化吡咯烷酮 BMT-415200 的替代方法
对映体纯 ((2 S ,3 S ,4 S )-3-ethyl-4-fluoro-5-oxopyrrolidin-2-yl)methyl methanesulfonate (BMT-415200) 1 的多千克规模合成路线的开发在这项工作中进行了描述。在这个序列中,实施了一个安全可靠的过程,该过程由九个线性步骤和四个隔离组成。该合成的特点是烯酮12的高度非对映选择性氢化、酮13的非对映选择性还原以及相应仲醇8的脱氧氟化,然后9被 C-H 氧化为内酰胺10。目标化合物1由市售二叔丁基 (S)-4-氧代吡咯烷-1,2-二羧酸酯 7 制备,总收率为 19% ,纯度> 99 %。
更新日期:2023-03-22
中文翻译:

大规模合成密集功能化吡咯烷酮 BMT-415200 的替代方法
对映体纯 ((2 S ,3 S ,4 S )-3-ethyl-4-fluoro-5-oxopyrrolidin-2-yl)methyl methanesulfonate (BMT-415200) 1 的多千克规模合成路线的开发在这项工作中进行了描述。在这个序列中,实施了一个安全可靠的过程,该过程由九个线性步骤和四个隔离组成。该合成的特点是烯酮12的高度非对映选择性氢化、酮13的非对映选择性还原以及相应仲醇8的脱氧氟化,然后9被 C-H 氧化为内酰胺10。目标化合物1由市售二叔丁基 (S)-4-氧代吡咯烷-1,2-二羧酸酯 7 制备,总收率为 19% ,纯度> 99 %。











































 京公网安备 11010802027423号
京公网安备 11010802027423号