当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Emerging Research Needs for Characterizing the Risks of Global Lithium Pollution under Carbon Neutrality Strategies
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-03-24 , DOI: 10.1021/acs.est.3c01431 Xuezhi Yang 1 , Haonan Wen 1 , Yue Lin 1, 2 , Haiyan Zhang 1 , Yin Liu 1 , Jianjie Fu 1, 2, 3 , Qian Liu 2, 3 , Guibin Jiang 1, 2, 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-03-24 , DOI: 10.1021/acs.est.3c01431 Xuezhi Yang 1 , Haonan Wen 1 , Yue Lin 1, 2 , Haiyan Zhang 1 , Yin Liu 1 , Jianjie Fu 1, 2, 3 , Qian Liu 2, 3 , Guibin Jiang 1, 2, 3
Affiliation
|
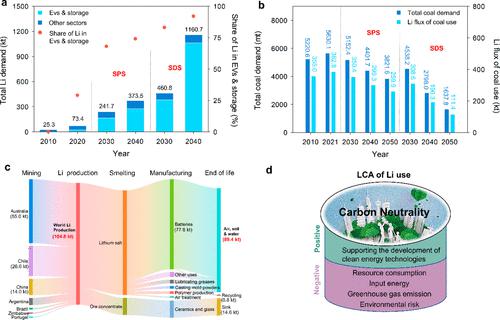
Lithium (Li) is a relatively rare element (27th in rank of elemental abundance), with a background concentration of 20–60 mg/kg in the upper earth’s crust. It is very difficult for Li to leach from the crystal lattice of most minerals and rocks, so little is dissolved to surface freshwater systems. In addition to weathering–leaching processes, the occurrence of Li in environmental media is also largely affected by human activities. In terms of the biogeochemical cycle, mobilization of Li from the upper earth’s crust by human activities (mainly Li mining, coal burning, and use of groundwater; >1000 kt/year) is estimated to be much greater than that by natural processes (mainly chemical and mechanical weathering and volcanic emission; 226 kt/year). (1) Currently, Li has been found to be widely distributed in environmental media such as air, water, and soil, which is potentially harmful to microorganisms, plants, animals, and humans when the concentration exceeds the safe threshold. (2−4) To date, although the anthropogenic inputs of Li in rivers and tap water in densely populated areas have been reported, (2) the human impacts on the Li levels in diverse environmental media remain largely unknown. To mitigate global warming, 195 countries cosigned an agreement in Paris in 2015 (i.e., Paris Agreement) and laid down a consensus that “carbon neutrality” by the mid-21st century is essential. (5) To pursue the carbon neutrality goal, a dramatic increase in Li production at the global scale is predicted, as lithium-ion batteries (LIBs) have become the key to the development and application of clean energy technologies [i.e., electric vehicles (EVs) and battery storage in electric grids]. Figure 1a shows the global Li demand by sector (i.e., EVs, storage, and other sectors) and scenario [i.e., stated policies scenario (SPS) and sustainable development scenario (SDS)] from 2020 to 2040. Between 2020 and 2040, the global demand for Li is estimated to grow from 73.4 to 373.5 kt/year (∼5 times) in SPS and to 1160.7 kt/year (∼40 times) in SDS. The EV and storage sectors account for 29% of the total Li demand in 2020 (up from a minuscule share in 2010), and the share is estimated to increase to 74% in the SPS and 92% in the SDS by 2040 due to the rapid deployment of EVs and energy storage under the background of carbon neutrality. On the contrary, as another important anthropogenic emission source of Li, coal combustion is expected to be controlled gradually (Figure 1b). Accordingly, between 2020 and 2050, the Li flux of coal use is estimated to decrease from 382.8 to 259.9 kt/year in SPS and to 111.4 kt/year in SDS. Overall, in light of the global Li flux of natural processes in the upper earth’s crust (226 kt/year), the perturbation of the global cycle of Li by human activities will vary remarkably during the implementation of the carbon neutrality strategy. Figure 1. Overview of global Li demand trends, global Li flux of coal use, global material flow of Li in industrial activities, and a framework of life cycle assessment (LCA) of Li use. (a) Global Li demand by sector and scenario from 2020 to 2040 [in kilotons, data from the International Energy Agency (IEA) (15)]. (b) Global Li flux of coal use in different scenarios from 2020 to 2050 (in million metric tons for coal and kilottons for Li). The estimates of Li flux were calculated by using the average Li concentration in globally produced coal (65 mg/kg) (1) computed form the relative contribution of coal mining in different countries and then multiplying by the historic and projected global coal consumption. Global coal consumption was derived from the ratio of the historic and projected global energy consumption from coal (in exajoules, data from the IEA (16)) and the caloric value of 1 kg of standard coal (29307.6 kJ/kg). In panels a and b, SPS refers to the stated policies scenario that reflects all of today’s announced policy intentions and targets, insofar as they are backed up by detailed measures for their realization, and SDS refers to the sustainable development scenario in which a surge in clean energy policies and investment puts the energy system on track to achieve sustainable energy objectives in full, including the Paris Agreement, energy access, and air quality goals. These two scenarios are taken from the projections in the World Energy Outlook 2020 (IEA). (17) (c) Global material flow of Li in industrial activities in 2021 (original data from Mineral Commodity Summaries 2022, U.S. Geological Survey (18)). (d) Framework of LCA of Li use under the background of carbon neutrality with regard to its positive and negative impacts. Furthermore, the carbon neutrality strategy will inevitably alter the routes of anthropogenic inputs of Li to the environment. The global end-use markets of Li in 2021 were estimated as follows: LIBs, 74%; ceramics and glass, 14%; lubricating greases, 3%; casting mold powders, 2%; polymer production, 2%; air treatment, 1%; and other uses, 4% (Figure 1c). LIBs were mainly used in EVs, energy storage fields, portable electronic devices, and electric tools, among which EVs and energy storage will drive the main global demand for LIBs in the next few decades (Figure 1a). In terms of global material flow, Li in industrial activities undergoes many processes (including mining, smelting, manufacturing, transportation, disposal, and recycling) and circulates in different regions of the world (Figure 1c). Although the Li-related industrial activities, especially the entire industrial chain for LIBs, are growing rapidly, little is known about the occupational exposure risks and the impact of anthropogenic input of Li to the environment in the relevant industrial areas. Moreover, Li-containing products are widely used in densely populated areas, and the disposal and landfill of these products may pose potential risks to urban residents all around the world. To date, the adverse health effects of Li are mainly evaluated on the basis of the clinically informative profile (because Li is widely used in the treatment of mood disorders) and corresponding animal studies (acute exposure at large doses), but subchronic or chronic animal exposure studies and environmentally relevant concentration exposure studies are still deficient. At large exposure doses, Li is found to be associated with an increased risk of hypothyroidism, hyperparathyroidism, reduced urinary concentrating ability, and weight gain. (6) Note that as a nutritionally nonessential metal, Li is found in nearly all organs of the human body, and the roles of Li in life activities must still be explored. In 2008, the U.S. Environmental Protection Agency proposed a provisional reference dose (RfD) of 2 μg kg–1 day–1 for Li in light of its adverse health effects. (7) On the basis of this value, the U.S. Geological Survey suggested a health-based screening level in drinking water of 10 μg/L and an upper limit of 60 μg/L. (7) However, the official regulatory value in drinking water and sewage discharge for Li is still not available. With the background mentioned above, intensive research efforts should address the following challenges for Li pollution. (1) Evaluating the integrative benefits of Li use with the goal of carbon neutrality based on life cycle assessment (LCA). To meet the demand of the rapid development of clean energy technologies (i.e., EVs and energy storage), more and more Li will be mined in the next few decades (Figure 1a), which will increase the environmental burden via the subsequent industrial activities (Figure 1c). As summarized in Figure 1d, the relevant environmental burdens include resource consumption (e.g., ore and water), input energy (e.g., electricity and fuel), greenhouse gas emission (e.g., CO2), and environmental risk (e.g., toxicity to microorganisms, plants, animals, and humans at environmentally relevant concentrations). For example, it has been estimated that 1 t of Li strip-mined in Chile was estimated to consume 540 t of water and release 5 t of CO2. (8) The LCA frameworks (including the cumulative energy demand method, the global warming potential method, the abiotic depletion potential method, the eco-indicator 99 method, etc.) will be effective tools for quantitatively evaluating the integrative benefits of Li use from different sides for optimizing global carbon neutrality strategies. However, the implementation of these LCA models, including the acquisition of more primary data in many industrial process (including the production data restricted by companies) and obtaining more toxicology data for Li at environmentally relevant concentrations, faces major challenges. (2) A more comprehensive understanding of the environmental and health impacts of Li pollution. Compared with that of heavy metals, such as Hg, Pb, and Cr, the toxicity of Li is normally thought to be much lower and thus attracts less attention. However, with the growing concern about Li pollution, its chronic and low-dose toxic effects on microorganisms, plants, animals, and humans need to be reevaluated. In addition to dissolved Li, the ecotoxicological effects of nanoscale Li-containing particles like LiCoO2 and LiFePO4 (widely used as cathode materials in LIBs) need to be further examined and unraveled. Furthermore, the environmental behaviors and fate of dissolved Li and Li-containing nanoparticles, including adsorption and partition between environmental interfaces, also need to be explored. With regard to the health risks, knowledge of the different Li exposure pathways (such as via ingestion, inhalation, or dermal contact) remains lacking, and its health risks at the population level are still unknown. With regard to pollution control, it has been found that Li cannot be removed by the current treatment processes of tap water and sewage, (2,9) so more effort should be dedicated to improve the treatment technologies with respect to Li, especially in Li-related industrial areas. In addition, further research on remediation technologies for Li contaminants on a field scale also needs to be conducted. (3) Tracing the sources and environmental processes of Li pollution in the environment. As shown in Figure 1c, most of the Li being mined will enter the environment at the end of the life of various Li-containing products, such as LIBs, lubricating greases, and medicine. An exception is the application in ceramics and glass (just like Li-containing rocks) in which Li is sparingly soluble, so they can be regarded as a sink of Li. Li release may occur in many scenarios, including mining, smelting, manufacturing, recycling, E-waste dismantling, and landfill. In addition, in densely populated areas like megacities, the disposal of Li-containing products and urban sewage may act as the major routes of anthropogenic input of Li to the urban environment. Tracing the sources and environmental processes of Li pollution is an important prerequisite for evaluating the human impacts and environmental risks. However, reliable tools for source tracing of Li pollution are still lacking. Some novel technologies, such as stable Li isotopic tracing, have shown great promise. It has been reported that Li in some products (including LIBs, grease, and medicine) has Li isotopic fingerprints distinguishable from that of naturally occurring Li. (2) In this regard, more effort should be dedicated to building representative Li isotopic fingerprint databases from different sources and gaining insight into the environmental processes of Li in the environment. (4) Improving LIB recycling techniques. Because LIBs are the major consumers of Li resources at present and will be in the future (Figure 1a,c), recycling Li from LIBs is beneficial to both resource conservation and pollution reduction. Although the recycling technology is now developing rapidly, (10−13) the global market of waste LIB recycling is still underdeveloped; e.g., <1% of Li is recycled in the European Union according to a report by the United Nations Environment Programme. (12,14) Li salts are the main components of the electrolytes of LIBs [e.g., lithium hexafluorophosphate (LiPF6)] and cathode materials, including lithium cobalt oxides (LiCoO2), lithium iron phosphate (LiFePO4), lithium manganate (LiMn2O4), NCM (LiNi1–2xCoxMnxO2), etc. Currently, the retrieval of high-value metals (e.g., Li, Co, Ni, and Mn) from these materials still faces many challenges, including how to improve the extraction efficiency, how to lower the costs and energy consumption, and how to reduce the environmental risks. For example, the currently used pyrometallurgical method is generally conducted at high temperatures (>700 °C) and may emit harmful acidic gases (i.e., HF), and the hydrometallurgical method requires a massive dose of inorganic acids and lengthy extraction steps. (10,11) Thus, more sustainable Li recovery techniques for LIBs are highly desired. Because LIBs are highly diverse in composition, type, and size in different regions and countries and the disposal guidelines for spent LIBs are still lacking, life cycle management for LIBs needs global coordination and joint efforts. Qian Liu is a Professor at the Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences (RCEES, CAS). He obtained his B.Sc. in 2004 and Ph.D. in 2009 from Hunan University. Thereafter, he obtained postdoc training at RCEES, CAS, in 2010–2012 and Trent University in 2013–2014. He became an Assistant Professor at RCEES, CAS, in 2012 and a Professor in 2017. Dr. Liu is a recipient of the National Science Fund for Distinguished Young Scholars and the NSFC Science Fund for Excellent Young Scholars. He has won the XPLORER Prize and MIT Technology Review Innovators Under 35 China. He now serves as the Executive Editor for Environment & Health and an Associate Editor for Environmental Science: Processes & Impacts. His research interests include environmental analytical chemistry, air pollution, exposomics, and effects of environmental pollution on health. This work was financially supported by the National Natural Science Foundation of China (21825403, 92143301, 22193052, and 22206034) and the Research Funds of Hangzhou Institute for Advanced Study (2022ZZ01019). This article references 18 other publications. This article has not yet been cited by other publications. Figure 1. Overview of global Li demand trends, global Li flux of coal use, global material flow of Li in industrial activities, and a framework of life cycle assessment (LCA) of Li use. (a) Global Li demand by sector and scenario from 2020 to 2040 [in kilotons, data from the International Energy Agency (IEA) (15)]. (b) Global Li flux of coal use in different scenarios from 2020 to 2050 (in million metric tons for coal and kilottons for Li). The estimates of Li flux were calculated by using the average Li concentration in globally produced coal (65 mg/kg) (1) computed form the relative contribution of coal mining in different countries and then multiplying by the historic and projected global coal consumption. Global coal consumption was derived from the ratio of the historic and projected global energy consumption from coal (in exajoules, data from the IEA (16)) and the caloric value of 1 kg of standard coal (29307.6 kJ/kg). In panels a and b, SPS refers to the stated policies scenario that reflects all of today’s announced policy intentions and targets, insofar as they are backed up by detailed measures for their realization, and SDS refers to the sustainable development scenario in which a surge in clean energy policies and investment puts the energy system on track to achieve sustainable energy objectives in full, including the Paris Agreement, energy access, and air quality goals. These two scenarios are taken from the projections in the World Energy Outlook 2020 (IEA). (17) (c) Global material flow of Li in industrial activities in 2021 (original data from Mineral Commodity Summaries 2022, U.S. Geological Survey (18)). (d) Framework of LCA of Li use under the background of carbon neutrality with regard to its positive and negative impacts. Qian Liu is a Professor at the Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences (RCEES, CAS). He obtained his B.Sc. in 2004 and Ph.D. in 2009 from Hunan University. Thereafter, he obtained postdoc training at RCEES, CAS, in 2010–2012 and Trent University in 2013–2014. He became an Assistant Professor at RCEES, CAS, in 2012 and a Professor in 2017. Dr. Liu is a recipient of the National Science Fund for Distinguished Young Scholars and the NSFC Science Fund for Excellent Young Scholars. He has won the XPLORER Prize and MIT Technology Review Innovators Under 35 China. He now serves as the Executive Editor for Environment & Health and an Associate Editor for Environmental Science: Processes & Impacts. His research interests include environmental analytical chemistry, air pollution, exposomics, and effects of environmental pollution on health. This article references 18 other publications.
更新日期:2023-03-24











































 京公网安备 11010802027423号
京公网安备 11010802027423号