Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2023-03-22 , DOI: 10.1016/j.jhazmat.2023.131267 Haoyu Luo 1 , Yi Wan 1 , Jie Li 1 , Yuhao Cai 1 , Zhi Dang 2 , Hua Yin 2
|
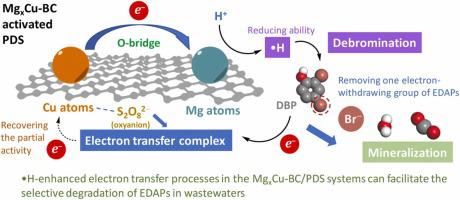
In wastewater treatment by persulfate-based advanced oxidation processes (PS-AOPs), electron-deficient aromatic pollutants (EDAPs) are refractory to nonradical pathway. To explore an efficient degradation pathway for EDAPs, MgxCu-biochar (BC) (x = 0.5, 1, 1.5) activated peroxydisulfate (PDS) was developed, which could trigger reductive species (•H) to reduce EDAPs first, and subsequently facilitate electron-transfer degradation of reduced intermediates. The roles of Mg-doping in MgxCu-BC to promote PDS activation and 2,4-dibromophenol (DBP) degradation were investigated. The mechanisms were then explored via electron paramagnetic resonance (EPR), chemical probes and Density Functional Theory (DFT) calculations. The results showed that Mg-doping improved metal-support interactions (MSIs) of MgxCu-BC, inducing •H formation via electron transfer from Cu atoms during PDS activation, which was thermodynamically favorable. The degradation rate of DBP (kobs, 0.0494 min–1) and Br– release (5.35 mg L–1) in Mg1Cu-BC systems were more 31 and 33 times than that in Cu-BC/PDS system, respectively. The degradation mechanism of •H-enhanced electron transfer processes was that •H attacked one Br group of DBP, and then debrominated intermediates were mineralized by electron transfer processes in the Mg1Cu-BC/PDS system. Overall, this study reports a novel pathway in PS-AOPs for selective degradation of EDAPs in wastewaters.
中文翻译:

MgxCu-biochar 活化的过二硫酸盐触发还原物质,用于还原和增强缺电子芳香族污染物的电子转移降解
在基于过硫酸盐的高级氧化工艺 (PS-AOPs) 的废水处理中,缺电子芳香族污染物 (EDAPs) 对非自由基途径具有抵抗力。为了探索 EDAPs 的有效降解途径,开发了 Mg x Cu-biochar (BC) (x = 0.5, 1, 1.5) 活化的过二硫酸盐 (PDS),它可以触发还原物质 (•H) 先还原 EDAPs,然后再还原促进还原中间体的电子转移降解。Mg掺杂在Mg x中的作用研究了促进 PDS 活化和 2,4-二溴苯酚 (DBP) 降解的 Cu-BC。然后通过电子顺磁共振 (EPR)、化学探针和密度泛函理论 (DFT) 计算探索这些机制。结果表明,Mg 掺杂改善了 Mg x Cu-BC 的金属-载体相互作用 (MSI),在 PDS 活化过程中通过 Cu 原子的电子转移诱导了 •H 的形成,这在热力学上是有利的。DBP ( k obs , 0.0494 min –1 ) 和 Br – release (5.35 mg L –1 ) 在 Mg 1中的降解率Cu-BC体系分别是Cu-BC/PDS体系的31倍和33倍。•H 增强电子转移过程的降解机制是•H 攻击DBP 的一个Br 基团,然后在Mg 1 Cu-BC/PDS 体系中通过电子转移过程将脱溴中间体矿化。总体而言,本研究报告了 PS-AOP 中选择性降解废水中 EDAP 的新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号