Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease ( IF 6.2 ) Pub Date : 2023-03-23 , DOI: 10.1016/j.bbadis.2023.166698 Liangwei Duan 1 , Yucong Zhao 1 , Jing Jia 1 , Tianzhu Chao 2 , Hao Wang 1 , Yinming Liang 1 , Yunwei Lou 1 , Qianqian Zheng 1 , Hui Wang 1
|
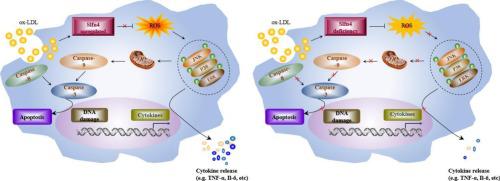
In atherosclerosis, macrophages derived from blood monocytes contribute to non-resolving inflammation, which subsequently primes necrotic core formation, and ultimately triggers acute thrombotic vascular disease. Nevertheless, little is known about how inflammatory cells, especially the macrophages fuel atherosclerosis. CD68, a unique class D scavenger receptor (SRD) family member, is specifically expressed in monocytes/macrophages and remarkably up-regulated upon oxidized low-density lipoprotein (ox-LDL) stimulation. Nonetheless, whether and how myeloid-specific CD68 affects atherosclerosis remains to be defined. To determine the essential in vivo role and mechanism linking CD68 to atherosclerosis, we engineered global and myeloid-specific CD68-deficient mice on an ApoE-null background. On Western diet, both the mice with global and the myeloid-restricted deletion of CD68 on ApoE-null background attenuated atherosclerosis, accompanied by diminished immune/inflammatory cell burden and necrotic core content, but increased smooth muscle cell content in atherosclerotic plaques. In vitro experiments revealed that CD68 deficiency in macrophages resulted in attenuated ox-LDL-induced macrophage apoptosis. Additionally, CD68 deficiency suppressed ROS production, while removal of ROS can markedly reversed this effect. We further showed that CD68 deficiency affected apoptosis through inactivation of the mitogen-activated protein kinase (MAPK) pathway. Our findings establish CD68 as a macrophage lineage-specific regulator of “ROS-MAPK-apoptosis” axis, thus providing a previously unknown mechanism for the prominence of CD68 as a risk factor for coronary artery disease. Its therapeutic inhibition may provide a potent lever to alleviate the cardiovascular disease.
中文翻译:

骨髓限制性 CD68 缺乏通过抑制 ROS-MAPK 凋亡轴减轻动脉粥样硬化
在动脉粥样硬化中,源自血液单核细胞的巨噬细胞导致无法消退的炎症,随后引发坏死核心形成,并最终引发急性血栓性血管疾病。然而,人们对炎症细胞,尤其是巨噬细胞如何促进动脉粥样硬化知之甚少。CD68 是一种独特的 D 类清道夫受体 (SRD) 家族成员,在单核细胞/巨噬细胞中特异性表达,并在氧化低密度脂蛋白 (ox-LDL) 刺激后显着上调。尽管如此,骨髓特异性 CD68 是否以及如何影响动脉粥样硬化仍有待确定。为了确定将 CD68 与动脉粥样硬化联系起来的重要体内作用和机制,我们在 ApoE 缺失的背景下设计了全球和骨髓特异性 CD68 缺陷小鼠。在西方饮食中,在 ApoE-null 背景下具有 CD68 的全局和骨髓限制性缺失的小鼠均减轻了动脉粥样硬化,伴随着免疫/炎症细胞负荷和坏死核心含量的减少,但动脉粥样硬化斑块中平滑肌细胞含量增加。体外实验表明,巨噬细胞中 CD68 的缺乏导致 ox-LDL 诱导的巨噬细胞凋亡减弱。此外,CD68 缺乏会抑制 ROS 的产生,而去除 ROS 可以显着逆转这种影响。我们进一步表明,CD68 缺乏通过丝裂原活化蛋白激酶 (MAPK) 通路的失活影响细胞凋亡。我们的研究结果将 CD68 确定为巨噬细胞谱系特异性调节剂“ROS-MAPK-凋亡”轴,从而为 CD68 作为冠状动脉疾病的危险因素的重要性提供了一个以前未知的机制。其治疗性抑制作用可能为减轻心血管疾病提供有效的杠杆。



























 京公网安备 11010802027423号
京公网安备 11010802027423号