Applied Surface Science ( IF 6.3 ) Pub Date : 2023-03-22 , DOI: 10.1016/j.apsusc.2023.157098 Yihai Zhou , Pin Song , Meng Pan , Hong Wang , Zhongying Liu , Jun Di , Daobin Liu , Jingxiang Low , Renchun Yang
|
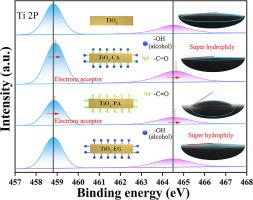
Photocatalytic H2 evolution from water splitting in liquid-solid two-phase requires an efficient photocatalyst with excellent ability of charge separation and reactant molecular diffusion. In this study, the super hydrophilic-electrons acceptor with abundant –C=O and –OH functional groups were evenly grafted onto rutile TiO2 nanorods surface to speed up the charge separation and water molecular diffusion, respectively. The photocatalytic results showed that the TiO2-CA grafted by citric acid (with three –C=O, three carboxy-(–OH) and one alcohol-(–OH)) presented a 1.48° contact angle and a distinct increased H2-production activity (48.5 mmol·h-1·g-1), whereas the TiO2-PA grafted by propane-1,2,3-tricarboxylic acid (Just lack of one alcohol-(–OH) compared with TiO2-CA) showed a 30.95° contact angle and a decreased H2-production activity (19.3 mmol·h-1·g-1) compared to the unmodified TiO2 (25.8 mmol·h-1·g-1), indicating that the super hydrophilic-electrons acceptor is vitally important to the photocatalytic activity of TiO2. Based on the present results, the carbonyl group (–C=O) possess a stronger reducing capability and act as “electrons acceptor”, while the only alcohol-hydroxyl group (–OH) in TiO2-CA can decrease the mass transfer resistance and serve as “super hydrophilic absorber”. Considering its mild preparation, inexpensive cost, and admirable efficiency, the super hydrophilic-electrons acceptor with dual functional groups regulated TiO2 can become a promising way for potential application in photocatalytic field.
中文翻译:

超亲水电子受体调节金红石二氧化钛纳米棒促进光催化产氢
液-固两相中水分解的光催化H 2释放需要具有优异的电荷分离和反应物分子扩散能力的高效光催化剂。 在本研究中,将具有丰富的–C=O 和–OH 官能团的超亲水电子受体均匀接枝到金红石TiO 2纳米棒表面,分别加速电荷分离和水分子扩散。光催化结果表明,柠檬酸接枝的TiO 2 -CA(三个-C=O,三个羧基-(-OH)和一个醇-(-OH))呈现出1.48°的接触角和明显增加的H 2 -生产活性(48.5 mmol·h -1 ·g -1),而TiO丙烷-1,2,3-三羧酸接枝的2 -PA(与 TiO 2 -CA 相比只缺少一种醇-(-OH) )显示出 30.95° 的接触角和降低的 H 2 -产生活性(19.3 mmol ·h -1 ·g -1 )与未改性的TiO 2 (25.8 mmol·h -1 ·g -1 )相比,表明超亲水电子受体对TiO 2的光催化活性至关重要。根据目前的结果,羰基(-C=O)具有更强的还原能力,充当“电子受体”,而TiO 2中唯一的醇羟基(-OH)-CA可以降低传质阻力,起到“超亲水吸收剂”的作用。考虑到其温和的制备、廉价的成本和令人钦佩的效率,具有双官能团调节的超亲水电子受体 TiO 2可能成为光催化领域潜在应用的一种有前途的方式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号