Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2023-03-22 , DOI: 10.1016/j.jhazmat.2023.131270 Andrei Veksha 1 , Yuxin Wang 2 , Jun Wei Foo 1 , Ichiro Naruse 3 , Grzegorz Lisak 4
|
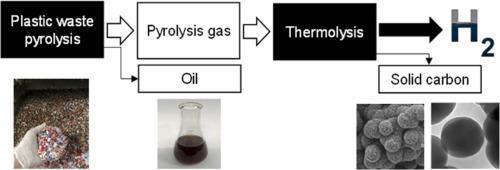
The replacement of natural gas with plastic-derived pyrolysis gas can defossilize H2 production, while subsequent capture, utilization and storage of carbon in a solid form can decarbonize the process. The objective of this study was to investigate H2 production from three types of plastics using a process comprising pyrolysis (600 °C) and thermolysis stages (1200–1500 °C). Depending on the plastic feedstock and thermolysis temperature, the laboratory-scale setup generated 1000–1350 mL/min product gas with H2 purity of 74.3–94.2 vol%. The recovery of 5–9 wt% molecular H2 per mass of plastics was achieved. Other products included solid residue (0.1–12 wt%) and oil (8–52 wt%) from the pyrolysis reactor, solid carbon (36–53 wt%) and gas impurities (2–16 wt%) from the thermolysis reactor. The purity of H2 gas was detrimentally influenced by polyethylene terephthalate in the feedstock due to the dilution of gas by CO. The decomposition of methane containing in the pyrolysis gas was the limiting reaction step during H2 production and improved at higher thermolysis temperature. Three solid carbon structures were formed during the thermolysis stage regardless of the plastic type: carbon black aggregates, carbon black aggregates coated with a layer of pyrolytic carbon and a carbon film on the inner reactor wall. Among the three types of carbon, the highest valorization potential was identified for carbon black aggregates. Plastic feedstock composition had little if any effect on carbon black properties, while high thermolysis temperature (1500 °C) reduced the particle sizes and increased the surface area of aggregates.
中文翻译:

废塑料制氢的去化石化和脱碳:热解阶段的温度和原料效应
用塑料衍生的热解气替代天然气可以使 H 2生产去化石化,而随后以固体形式捕获、利用和储存碳可以使该过程脱碳。本研究的目的是研究使用包括热解 (600 °C) 和热解阶段 (1200–1500 °C) 的过程从三种类型的塑料中生产H 2 。根据塑料原料和热解温度,实验室规模的设置产生了 1000–1350 mL/min 的产品气体,H 2纯度为 74.3–94.2 vol%。5–9 wt% 分子 H 2的回收率达到每质量塑料。其他产品包括来自热解反应器的固体残渣(0.1-12 wt%)和油(8-52 wt%),来自热解反应器的固体碳(36-53 wt%)和气体杂质(2-16 wt%)。由于气体被 CO稀释,原料中的聚对苯二甲酸乙二醇酯对 H 2 气体的纯度产生不利影响。热解气体中甲烷的分解是 H 2 过程中的限制性反应步骤生产和提高在较高的热解温度。无论塑料类型如何,在热解阶段都会形成三种固体碳结构:炭黑聚集体、涂有一层热解碳的炭黑聚集体和反应器内壁上的碳膜。在三种类型的碳中,炭黑聚集体的增值潜力最高。塑料原料组成对炭黑性能几乎没有影响,而高热解温度 (1500 °C) 会减小颗粒尺寸并增加聚集体的表面积。











































 京公网安备 11010802027423号
京公网安备 11010802027423号