当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamically Reversible Interconversion of Molecular Catalysts for Efficient Electrooxidation of Propylene into Propylene Glycol
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-03-21 , DOI: 10.1021/jacs.3c00660 Jingwen Ke 1 , Mingfang Chi 1 , Jiankang Zhao 1 , Yan Liu 1 , Ruyang Wang 1 , Kaiyuan Fan 1 , Yuxuan Zhou 1 , Zhikai Xi 1 , Xiangdong Kong 1 , Hongliang Li 1 , Jie Zeng 1, 2 , Zhigang Geng 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-03-21 , DOI: 10.1021/jacs.3c00660 Jingwen Ke 1 , Mingfang Chi 1 , Jiankang Zhao 1 , Yan Liu 1 , Ruyang Wang 1 , Kaiyuan Fan 1 , Yuxuan Zhou 1 , Zhikai Xi 1 , Xiangdong Kong 1 , Hongliang Li 1 , Jie Zeng 1, 2 , Zhigang Geng 1
Affiliation
|
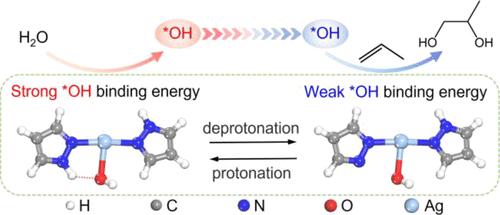
For the electrooxidation of propylene into 1,2-propylene glycol (PG), the process involves two key steps of the generation of *OH and the transfer of *OH to the C═C bond in propylene. The strong *OH binding energy (EB(*OH)) favors the dissociation of H2O into *OH, whereas the transfer of *OH to propylene will be impeded. The scaling relationship of the EB(*OH) plays a key role in affecting the catalytic performance toward propylene electrooxidation. Herein, we adopt an immobilized Ag pyrazole molecular catalyst (denoted as AgPz) as the electrocatalyst. The pyrrolic N–H in AgPz could undergo deprotonation to form pyrrolic N (denoted as AgPz-Hvac), which can be protonated reversibly. During propylene electrooxidation, the strong EB(*OH) on AgPz favors the dissociation of H2O into *OH. Subsequently, the AgPz transforms into AgPz-Hvac that possesses weak EB(*OH), benefiting to the further combination of *OH and propylene. The dynamically reversible interconversion between AgPz and AgPz-Hvac accompanied by changeable EB(*OH) breaks the scaling relationship, thus greatly lowering the reaction barrier. At 2.0 V versus Ag/AgCl electrode, AgPz achieves a remarkable yield rate of 288.9 mmolPG gcat–1 h–1, which is more than one order of magnitude higher than the highest value ever reported.
中文翻译:

用于丙烯高效电氧化成丙二醇的分子催化剂的动态可逆互变
丙烯电氧化生成1,2-丙二醇(PG)过程包括*OH的生成和*OH向丙烯中C=C键的转移两个关键步骤。强*OH 结合能 ( E B (*OH)) 有利于 H 2 O离解成 *OH,而 *OH 向丙烯的转移将受到阻碍。E B (*OH)的比例关系在影响丙烯电氧化的催化性能方面起着关键作用。在此,我们采用固定化银吡唑分子催化剂(表示为 AgPz)作为电催化剂。AgPz 中的吡咯 N-H 可以去质子化形成吡咯 N(表示为 AgPz-H vac), 它可以被可逆地质子化。在丙烯电氧化过程中,AgPz 上的强E B (*OH) 有利于 H 2 O离解成 *OH。随后,AgPz转化为具有弱E B (*OH)的AgPz-H vac,有利于*OH与丙烯的进一步结合。AgPz 和 AgPz-H vac之间的动态可逆相互转化伴随着可变的E B (*OH) 打破了标度关系,从而大大降低了反应势垒。在 2.0 V 相对于 Ag/AgCl 电极时,AgPz 实现了 288.9 mmol PG g cat –1 h –1的惊人产率,这比有史以来报告的最高值高出一个数量级以上。
更新日期:2023-03-21
中文翻译:

用于丙烯高效电氧化成丙二醇的分子催化剂的动态可逆互变
丙烯电氧化生成1,2-丙二醇(PG)过程包括*OH的生成和*OH向丙烯中C=C键的转移两个关键步骤。强*OH 结合能 ( E B (*OH)) 有利于 H 2 O离解成 *OH,而 *OH 向丙烯的转移将受到阻碍。E B (*OH)的比例关系在影响丙烯电氧化的催化性能方面起着关键作用。在此,我们采用固定化银吡唑分子催化剂(表示为 AgPz)作为电催化剂。AgPz 中的吡咯 N-H 可以去质子化形成吡咯 N(表示为 AgPz-H vac), 它可以被可逆地质子化。在丙烯电氧化过程中,AgPz 上的强E B (*OH) 有利于 H 2 O离解成 *OH。随后,AgPz转化为具有弱E B (*OH)的AgPz-H vac,有利于*OH与丙烯的进一步结合。AgPz 和 AgPz-H vac之间的动态可逆相互转化伴随着可变的E B (*OH) 打破了标度关系,从而大大降低了反应势垒。在 2.0 V 相对于 Ag/AgCl 电极时,AgPz 实现了 288.9 mmol PG g cat –1 h –1的惊人产率,这比有史以来报告的最高值高出一个数量级以上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号