当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inhibition of PINK1-Mediated Mitophagy Contributes to Postoperative Cognitive Dysfunction through Activation of Caspase-3/GSDME-Dependent Pyroptosis
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2023-03-22 , DOI: 10.1021/acschemneuro.2c00691 Wei Wang 1 , Bo Zhao 1 , Wenwei Gao 2 , Wenqin Song 1 , Jiabao Hou 1 , Lei Zhang 1 , Zhongyuan Xia 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2023-03-22 , DOI: 10.1021/acschemneuro.2c00691 Wei Wang 1 , Bo Zhao 1 , Wenwei Gao 2 , Wenqin Song 1 , Jiabao Hou 1 , Lei Zhang 1 , Zhongyuan Xia 1
Affiliation
|
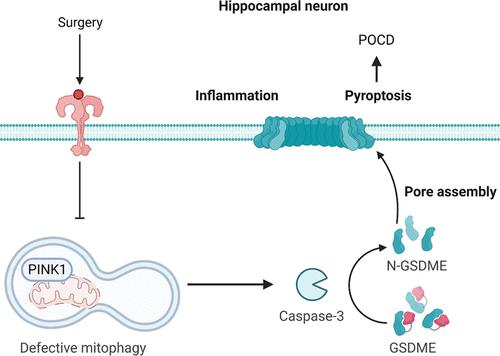
PTEN-induced kinase 1 (PINK1)-mediated mitophagy and caspase-1/gasdermin D canonical pyroptosis pathways have been implicated in the pathogenesis of postoperative cognitive dysfunction (POCD). However, gasdermin E (GSDME), another recently identified executioner of pyroptosis that can be specifically cleaved by caspase-3, is highly expressed in the brain and neurons. This study aimed to ascertain whether PINK1-dependent mitophagy governs postoperative cognitive capacity through caspase-3/GSDME. Twelve month old male Sprague-Dawley rats underwent exploratory laparotomy under isoflurane anesthesia. Lipopolysaccharide (LPS)-primed SH-SY5Y cells were used to mimic postsurgical neuroinflammation. For the interventional study, rats were administered with adeno-associated virus serotype 9 (AAV9)-mediated silencing of Pink1 and/or caspase-3 inhibitor Ac-DEVD-CHO (Ac-DC). SH-SY5Y cells were treated with siPINK1 and/or Ac-DC. Cognitive performance was assessed using the Morris water maze test. The mitophagy- and pyroptosis-related parameters were determined in the hippocampus and SH-SY5Y cells. Anesthesia/surgery and LPS caused defective PINK1-mediated mitophagy and activation of caspase-3/GSDME-dependent pyroptosis. AAV-9 mediated Pink1 overexpression mitigated cognitive impairment and caspase-3/GSDME-dependent pyroptosis. Conversely, inhibition of PINK1 aggravates POCD and overactivates neuronal pyroptosis. These abnormalities were rescued by Ac-DC treatment. Collectively, PINK1-mediated mitophagy regulates anesthesia and surgery-induced cognitive impairment by negatively affecting the caspase-3/GSDME pyroptosis pathway, which provides a promising therapeutic target for POCD.
中文翻译:

抑制 PINK1 介导的线粒体自噬通过激活 Caspase-3/GSDME 依赖性细胞焦亡导致术后认知功能障碍
PTEN 诱导的激酶 1 (PINK1) 介导的线粒体自噬和 caspase-1/gasdermin D 经典细胞焦亡途径与术后认知功能障碍 (POCD) 的发病机制有关。然而,gasdermin E (GSDME) 是另一种最近发现的焦亡执行者,可以被 caspase-3 特异性切割,在大脑和神经元中高度表达。本研究旨在确定 PINK1 依赖性线粒体自噬是否通过 caspase-3/GSDME 控制术后认知能力。十二个月大的雄性 Sprague-Dawley 大鼠在异氟醚麻醉下接受剖腹探查术。脂多糖(LPS)引发的 SH-SY5Y 细胞用于模拟术后神经炎症。在介入研究中,大鼠接受了腺相关病毒血清型 9 (AAV9) 介导的 Pink1 沉默和/或 caspase-3 抑制剂 Ac-DEVD-CHO (Ac-DC)。SH-SY5Y 细胞用 siPINK1 和/或 Ac-DC 处理。使用莫里斯水迷宫测试评估认知表现。在海马和 SH-SY5Y 细胞中测定线粒体自噬和细胞焦亡相关参数。麻醉/手术和 LPS 导致 PINK1 介导的线粒体自噬缺陷和 caspase-3/GSDME 依赖性细胞焦亡的激活。AAV-9 介导的 Pink1 过度表达可减轻认知障碍和 caspase-3/GSDME 依赖性细胞焦亡。相反,抑制 PINK1 会加重 POCD 并过度激活神经元焦亡。这些异常通过 Ac-DC 治疗得以挽救。总的来说,PINK1 介导的线粒体自噬通过对 caspase-3/GSDME 焦亡通路产生负面影响来调节麻醉和手术引起的认知障碍,这为 POCD 提供了一个有前途的治疗靶点。
更新日期:2023-03-22
中文翻译:

抑制 PINK1 介导的线粒体自噬通过激活 Caspase-3/GSDME 依赖性细胞焦亡导致术后认知功能障碍
PTEN 诱导的激酶 1 (PINK1) 介导的线粒体自噬和 caspase-1/gasdermin D 经典细胞焦亡途径与术后认知功能障碍 (POCD) 的发病机制有关。然而,gasdermin E (GSDME) 是另一种最近发现的焦亡执行者,可以被 caspase-3 特异性切割,在大脑和神经元中高度表达。本研究旨在确定 PINK1 依赖性线粒体自噬是否通过 caspase-3/GSDME 控制术后认知能力。十二个月大的雄性 Sprague-Dawley 大鼠在异氟醚麻醉下接受剖腹探查术。脂多糖(LPS)引发的 SH-SY5Y 细胞用于模拟术后神经炎症。在介入研究中,大鼠接受了腺相关病毒血清型 9 (AAV9) 介导的 Pink1 沉默和/或 caspase-3 抑制剂 Ac-DEVD-CHO (Ac-DC)。SH-SY5Y 细胞用 siPINK1 和/或 Ac-DC 处理。使用莫里斯水迷宫测试评估认知表现。在海马和 SH-SY5Y 细胞中测定线粒体自噬和细胞焦亡相关参数。麻醉/手术和 LPS 导致 PINK1 介导的线粒体自噬缺陷和 caspase-3/GSDME 依赖性细胞焦亡的激活。AAV-9 介导的 Pink1 过度表达可减轻认知障碍和 caspase-3/GSDME 依赖性细胞焦亡。相反,抑制 PINK1 会加重 POCD 并过度激活神经元焦亡。这些异常通过 Ac-DC 治疗得以挽救。总的来说,PINK1 介导的线粒体自噬通过对 caspase-3/GSDME 焦亡通路产生负面影响来调节麻醉和手术引起的认知障碍,这为 POCD 提供了一个有前途的治疗靶点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号