Structure ( IF 4.4 ) Pub Date : 2023-03-21 , DOI: 10.1016/j.str.2023.02.012 Burak T. Kaynak , Zakaria L. Dahmani , Pemra Doruker , Anupam Banerjee , Shang-Hua Yang , Reuven Gordon , Laura S. Itzhaki , Ivet Bahar
|
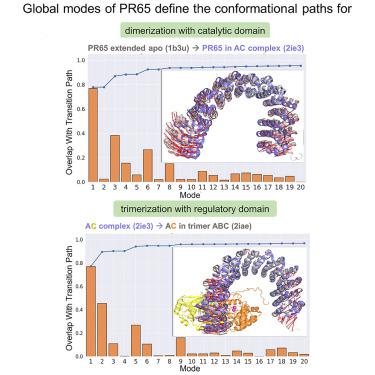
PR65, a horseshoe-shaped scaffold composed of 15 HEAT (observed in Huntingtin, elongation factor 3, protein phosphatase 2A, and the yeast kinase TOR1) repeats, forms, together with catalytic and regulatory subunits, the heterotrimeric protein phosphatase PP2A. We examined the role of PR65 in enabling PP2A enzymatic activity with computations at various levels of complexity, including hybrid approaches that combine full-atomic and elastic network models. Our study points to the high flexibility of this scaffold allowing for end-to-end distance fluctuations of 40–50 Å between compact and extended conformations. Notably, the intrinsic dynamics of PR65 facilitates complexation with the catalytic subunit and is retained in the PP2A complex enabling PR65 to engage the two domains of the catalytic subunit and provide the mechanical framework for enzymatic activity, with support from the regulatory subunit. In particular, the intra-repeat coils at the C-terminal arm play an important role in allosterically mediating the collective dynamics of PP2A, pointing to target sites for modulating PR65 function.
中文翻译:

PR65支架的协同机制是磷酸酶PP2A变构调节的基础
PR65 是一种马蹄形支架,由 15 个 HEAT(在亨廷顿蛋白、延伸因子 3、蛋白磷酸酶 2A 和酵母激酶 TOR1 中观察到)重复组成,与催化和调节亚基一起形成异三聚体蛋白磷酸酶 PP2A。我们研究了 PR65 在通过不同复杂程度的计算实现 PP2A 酶活性方面的作用,包括结合全原子和弹性网络模型的混合方法。我们的研究表明,该支架具有高度灵活性,允许紧凑构象和延伸构象之间的端到端距离波动为 40-50 Å。值得注意的是,PR65 的内在动力学促进与催化亚基的络合,并保留在 PP2A 复合物中,使 PR65 能够与催化亚基的两个结构域结合,并在调节亚基的支持下为酶活性提供机械框架。特别是,C 端臂的重复内线圈在变构介导 PP2A 的集体动力学中发挥着重要作用,指向调节 PR65 功能的目标位点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号