Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-03-20 , DOI: 10.1016/j.cej.2023.142513 Wen-luan Xie , Bin Hu , Yuan-gu Xia , Guo-yong Song , Ji Liu , Ying Liu , Qiang Lu
|
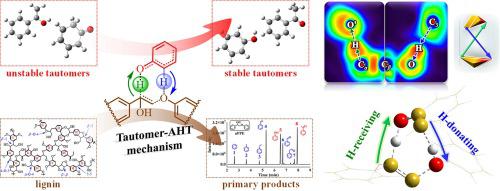
Bimolecular concerted interaction mechanism plays a vital role in lignin pyrolysis. According to the recently proposed hydroxyl-assisted hydrogen transfer (AHT) mechanism, the primary pyrolytic products can assist the hydrogen transfer process of linkage decomposition with their hydroxy groups acting as the mediator. Herein, the concerted interaction between two hydrogen transfer processes was further studied for the β-O-4 linked lignin. A novel concerted interaction mechanism named tautomer-AHT was found and carefully confirmed by combining theoretical calculations, electronic analyses, pyrolysis and isotope experiments, with 2-phenoxy-1-phenylethanol (α-OH-PPE) as the β-O-4 lignin model compound. During this interaction, the tautomer can make the best of its saturated and unsaturated groups to carry out hydrogen-donating and hydrogen-receiving processes of the β-O-4 breakage, respectively, accompanied by the hydrogen transfer of the mediator itself. As a result, the β-O-4 breakage can be promoted and the unstable tautomer turns into its corresponding stable isomer, leading to the formation of stable phenolics and ketones. In the early pyrolysis stage, the tautomer-AHT reaction triggered by unstable tautomers is basically superior to the hydroxyl-AHT reaction due to the lower energy barrier. With the consumption of unstable tautomers and the accumulation of stable hydroxy products, hydroxyl-AHT interaction becomes predominant in the latter pyrolysis stage. The bimolecular tautomer-AHT mechanism builds a special relationship between hydrogen transfer reactions, and lays a theoretical foundation for exploring complex interactions in lignin pyrolysis.
中文翻译:

木质素热解中双分子协同相互作用的新见解:互变异构体辅助氢转移机制
双分子协同相互作用机制在木质素热解中起着至关重要的作用。根据最近提出的羟基辅助氢转移(AHT)机制,初级热解产物可以以其羟基作为介体辅助键分解的氢转移过程。在此,针对β -O-4 连接的木质素,进一步研究了两个氢转移过程之间的协同相互作用。通过结合理论计算、电子分析、热解和同位素实验,发现并仔细证实了一种名为互变异构体-AHT 的新型协同相互作用机制,其中 2-苯氧基-1-苯乙醇 ( α -OH-PPE )作为β-O-4 木质素模型化合物。在这种相互作用过程中,互变异构体可以充分利用其饱和基团和不饱和基团分别进行β-O- 4断裂的供氢和受氢过程,同时伴随着介体本身的氢转移。结果,β可以促进-O-4的断裂,不稳定的互变异构体转变为相应的稳定异构体,从而形成稳定的酚类和酮类。在早期热解阶段,不稳定互变异构体引发的互变异构体-AHT反应由于能垒较低,基本上优于羟基-AHT反应。随着不稳定互变异构体的消耗和稳定羟基产物的积累,羟基-AHT相互作用在后期热解阶段成为主导。双分子互变异构体-AHT机制在氢转移反应之间建立了特殊的关系,为探索木质素热解中的复杂相互作用奠定了理论基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号