当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-Substrate Helical Character Matching Determines the Enantioselectivity in the Ishihara-Type Iodoarenes Catalyzed Asymmetric Kita-Dearomative Spirolactonization
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-03-20 , DOI: 10.1021/jacs.2c13295 Hanliang Zheng 1 , Liu Cai 2 , Ming Pan 2 , Muhammet Uyanik 3 , Kazuaki Ishihara 3 , Xiao-Song Xue 2, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-03-20 , DOI: 10.1021/jacs.2c13295 Hanliang Zheng 1 , Liu Cai 2 , Ming Pan 2 , Muhammet Uyanik 3 , Kazuaki Ishihara 3 , Xiao-Song Xue 2, 4
Affiliation
|
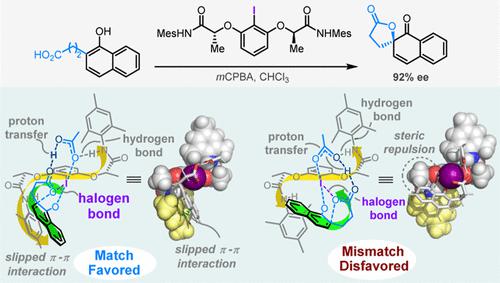
Catalyst design has traditionally focused on rigid structural elements to prevent conformational flexibility. Ishihara’s elegant design of conformationally flexible C2-symmetric iodoarenes, a new class of privileged organocatalysts, for the catalytic asymmetric dearomatization (CADA) of naphthols is a notable exception. Despite the widespread use of the Ishihara catalysts for CADAs, the reaction mechanism remains the subject of debate, and the mode of asymmetric induction has not been well established. Here, we report an in-depth computational investigation of three possible mechanisms in the literature. Our results, however, reveal that this reaction is best rationalized by a fourth mechanism called “proton-transfer-coupled-dearomatization (PTCD)”, which is predicted to be strongly favored over other competing pathways. The PTCD mechanism is consistent with a control experiment and further validated by applying it to rationalize the enantioselectivities. Oxidation of the flexible I(I) catalyst to catalytic active I(III) species induces a defined C2-symmetric helical chiral environment with a delicate balance between flexibility and rigidity. A match/mismatch effect between the active catalyst and the substrate’s helical shape in the dearomatization transition states was observed. The helical shape match allows the active catalyst to adapt its conformation to maximize attractive noncovalent interactions, including I(III)···O halogen bond, N–H···O hydrogen bond, and π···π stacking, to stabilize the favored transition state. A stereochemical model capable of rationalizing the effect of catalyst structural variation on the enantioselectivities is developed. The present study enriches our understanding of how flexible catalysts achieve high stereoinduction and may serve as an inspiration for the future exploration of conformational flexibility for new catalyst designs.
中文翻译:

催化剂-底物螺旋特征匹配决定了 Ishihara 型碘代芳烃催化的不对称 Kita-脱芳烃螺内酯化反应中的对映选择性
催化剂设计传统上侧重于刚性结构元素,以防止构象的灵活性。Ishihara 优雅的构象柔性C 2设计- 对称碘芳烃是一类新的特殊有机催化剂,用于萘酚的催化不对称脱芳构化 (CADA) 是一个显着的例外。尽管 Ishihara 催化剂广泛用于 CADA,但反应机制仍然存在争议,不对称诱导模式尚未确定。在这里,我们报告了对文献中三种可能机制的深入计算调查。然而,我们的结果表明,这种反应最好通过称为“质子转移偶联脱芳构化 (PTCD)”的第四种机制合理化,预计这种机制比其他竞争途径更受青睐。PTCD 机制与对照实验一致,并通过应用它使对映选择性合理化进一步验证。2个-对称的螺旋手性环境,在灵活性和刚性之间达到微妙的平衡。观察到活性催化剂与底物在脱芳构化过渡态中的螺旋形状之间的匹配/不匹配效应。螺旋形状匹配允许活性催化剂调整其构象以最大化有吸引力的非共价相互作用,包括 I(III)···O 卤素键、N–H···O 氢键和 π···π 堆叠,以稳定受青睐的过渡状态。开发了一种能够合理化催化剂结构变化对对映选择性影响的立体化学模型。本研究丰富了我们对柔性催化剂如何实现高立体诱导的理解,并可能为未来探索新催化剂设计的构象柔性提供灵感。
更新日期:2023-03-20
中文翻译:

催化剂-底物螺旋特征匹配决定了 Ishihara 型碘代芳烃催化的不对称 Kita-脱芳烃螺内酯化反应中的对映选择性
催化剂设计传统上侧重于刚性结构元素,以防止构象的灵活性。Ishihara 优雅的构象柔性C 2设计- 对称碘芳烃是一类新的特殊有机催化剂,用于萘酚的催化不对称脱芳构化 (CADA) 是一个显着的例外。尽管 Ishihara 催化剂广泛用于 CADA,但反应机制仍然存在争议,不对称诱导模式尚未确定。在这里,我们报告了对文献中三种可能机制的深入计算调查。然而,我们的结果表明,这种反应最好通过称为“质子转移偶联脱芳构化 (PTCD)”的第四种机制合理化,预计这种机制比其他竞争途径更受青睐。PTCD 机制与对照实验一致,并通过应用它使对映选择性合理化进一步验证。2个-对称的螺旋手性环境,在灵活性和刚性之间达到微妙的平衡。观察到活性催化剂与底物在脱芳构化过渡态中的螺旋形状之间的匹配/不匹配效应。螺旋形状匹配允许活性催化剂调整其构象以最大化有吸引力的非共价相互作用,包括 I(III)···O 卤素键、N–H···O 氢键和 π···π 堆叠,以稳定受青睐的过渡状态。开发了一种能够合理化催化剂结构变化对对映选择性影响的立体化学模型。本研究丰富了我们对柔性催化剂如何实现高立体诱导的理解,并可能为未来探索新催化剂设计的构象柔性提供灵感。











































 京公网安备 11010802027423号
京公网安备 11010802027423号