当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the Pore Size-Dependent Sorption Mechanism of Toluene and Cetane in Porous Carbon by Nuclear Magnetic Resonance
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-03-17 , DOI: 10.1021/acs.est.2c07086 Zhiang Hou 1 , Xiaoqiang An 1 , Kai Zhu 1, 2 , Qingwen Tang 1 , Huachun Lan 1 , Huijuan Liu 1 , Jiuhui Qu 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-03-17 , DOI: 10.1021/acs.est.2c07086 Zhiang Hou 1 , Xiaoqiang An 1 , Kai Zhu 1, 2 , Qingwen Tang 1 , Huachun Lan 1 , Huijuan Liu 1 , Jiuhui Qu 1
Affiliation
|
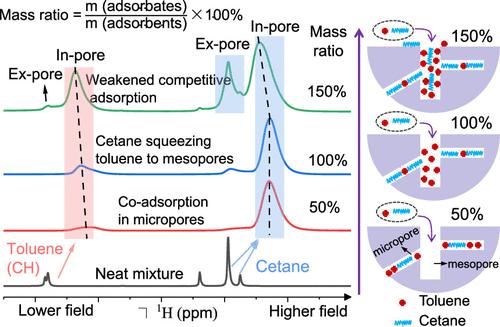
The adsorption of contaminants by porous carbon has been extensively studied by conventional isotherm and kinetic methods. However, the co-adsorption behavior and sorption sites of multiple contaminants in different-sized pores remain unclear. Herein, the nuclear magnetic resonance (NMR) approach is performed to investigate the adsorption mechanism of toluene and cetane in the confined space of carbon at the molecular level. The ring current effect induces the variation in the NMR chemical shifts of in-pore adsorbed toluene and cetane, realizing the identification of pore-dependent adsorption sites for contaminant removal. Cetane has a slower adsorption kinetic but a higher binding energy than toluene, which could squeeze toluene from micropores to larger pores with increasing adsorption quantity. This leads to a stronger competitive adsorption effect in small micropores than in mesopores. Accordingly, hierarchical porous carbons are determined to be the most effective adsorbents for the adsorption of coexisting contaminants. This study not only provides an effective NMR method to reveal the adsorption mechanism in the confined space of porous carbon at the molecular level but also offers new insights into the pore size-dependent adsorption of activated carbon for petroleum contaminant treatment.
中文翻译:

通过核磁共振揭示多孔碳中甲苯和十六烷的孔径依赖吸附机理
已经通过传统的等温线和动力学方法广泛研究了多孔碳对污染物的吸附。然而,多种污染物在不同尺寸孔隙中的共吸附行为和吸附位点仍不清楚。在此,采用核磁共振 (NMR) 方法在分子水平上研究甲苯和十六烷在碳的受限空间中的吸附机理。环电流效应引起孔内吸附的甲苯和十六烷的 NMR 化学位移的变化,实现了孔依赖吸附位点的识别以去除污染物。与甲苯相比,十六烷具有较慢的吸附动力学但具有较高的结合能,这可以随着吸附量的增加将甲苯从微孔挤压到较大的孔。这导致在小微孔中比在中孔中具有更强的竞争吸附效应。因此,分级多孔碳被确定为吸附共存污染物的最有效吸附剂。该研究不仅提供了一种有效的核磁共振方法来在分子水平上揭示多孔碳在受限空间中的吸附机理,而且为活性炭的孔径依赖性吸附用于石油污染物处理提供了新的见解。
更新日期:2023-03-17
中文翻译:

通过核磁共振揭示多孔碳中甲苯和十六烷的孔径依赖吸附机理
已经通过传统的等温线和动力学方法广泛研究了多孔碳对污染物的吸附。然而,多种污染物在不同尺寸孔隙中的共吸附行为和吸附位点仍不清楚。在此,采用核磁共振 (NMR) 方法在分子水平上研究甲苯和十六烷在碳的受限空间中的吸附机理。环电流效应引起孔内吸附的甲苯和十六烷的 NMR 化学位移的变化,实现了孔依赖吸附位点的识别以去除污染物。与甲苯相比,十六烷具有较慢的吸附动力学但具有较高的结合能,这可以随着吸附量的增加将甲苯从微孔挤压到较大的孔。这导致在小微孔中比在中孔中具有更强的竞争吸附效应。因此,分级多孔碳被确定为吸附共存污染物的最有效吸附剂。该研究不仅提供了一种有效的核磁共振方法来在分子水平上揭示多孔碳在受限空间中的吸附机理,而且为活性炭的孔径依赖性吸附用于石油污染物处理提供了新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号