Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2023-03-16 , DOI: 10.1016/j.jhazmat.2023.131210 Ningchao Zheng 1 , Xinhui Tang 1 , Yekai Lian 1 , Zheshun Ou 1 , Quan Zhou 1 , Ruilin Wang 1 , Zhuofeng Hu 1
|
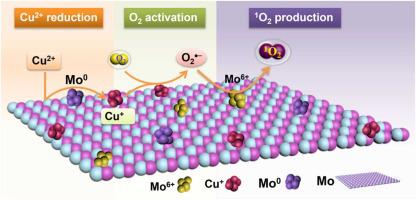
Singlet oxygen (1O2), which is difficult to generate, plays an important role in chemosynthesis, biomedicine and environment. Molecular oxygen (O2) is a green oxidant to produce 1O2 cost-effectively. However, O2 activation is difficult due to its spin-forbidden nature. Moreover, the main products of O2 activation are basically hydrogen peroxide (H2O2) and hydroxyl radical (•OH), but rarely 1O2. Herein, we innovatively realize the selective generation of 1O2 via O2 activation by a facile molybdenum (Mo)/Cu2+ system. In this system, Mo firstly reduces Cu2+ in solution to low-valence Cu0/Cu+ on its surface. Cu0/Cu+ activates O2 to generate superoxide radical (O2•−). Importantly, O2•− can be captured immediately and oxidized to 1O2 by surface-bound Mo6+ rather than reduced to H2O2. As a result, the Mo/Cu2+ system can selectively produce 1O2. Under air and O2 conditions, the degradation efficiency of ibuprofen by Mo/Cu2+ system is 67.2 % and 76.6 %, respectively. The degradation efficiencies of bisphenol A, rhodamine B and furfuryl alcohol are 77.1 %, 87.7 % and 91.1 %, respectively. The dosages of Mo and Cu2+ are 0.4 g/L and 3 mM, respectively, and the reaction time is 2 h. Interestingly, the activity of Mo decreased by only 4.2 % after 4 cycles. Therefore, this study provides a green pathway to selectively generate 1O2 for advanced oxidation processes.
中文翻译:

钼上的低价铜触发分子氧活化以选择性地产生用于高级氧化过程的单线态氧
难以生成的单线态氧( 1 O 2 )在化学合成、生物医学和环境中起着重要作用。分子氧 (O 2 ) 是一种绿色氧化剂,可以经济高效地生产1 O 2 。然而,O 2激活由于其自旋禁止的性质是困难的。此外,O 2活化的主要产物基本上是过氧化氢(H 2 O 2 )和羟基自由基(•OH),很少有1 O 2。在此,我们创新性地实现了O 2选择性生成1 O 2通过简单的钼 (Mo)/Cu 2+系统激活。在该体系中,Mo首先将溶液中的Cu 2+还原成其表面的低价Cu 0 /Cu + 。Cu 0 /Cu +活化O 2产生超氧自由基(O 2 •- )。重要的是,O 2 •-可以被表面结合的Mo 6+立即捕获并氧化成1 O 2而不是还原成H 2 O 2。因此,Mo/Cu 2+系统可以选择性地产生1 O 2. 在空气和O 2条件下,Mo/Cu 2+体系对布洛芬的降解效率分别为67.2 %和76.6 %。双酚A、罗丹明B和糠醇的降解效率分别为77.1%、87.7%和91.1%。Mo和Cu 2+的用量分别为0.4 g/L和3 mM,反应时间为2 h。有趣的是,Mo 的活性在 4 个循环后仅下降了 4.2%。因此,本研究为高级氧化过程选择性生成1 O 2提供了一条绿色途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号