Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2023-03-15 , DOI: 10.1016/j.jhazmat.2023.131217 Minghao Ma 1 , Ruixia Wang 1 , Ming Xu 2
|
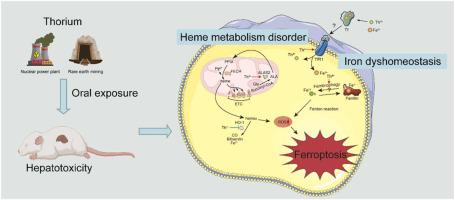
Thorium is a byproduct of the rare earth mining industry and can be utilized as fuel for the next-generation nuclear power facilities, which may pose health risks to the population. Although published literature has shown that the toxicity of thorium possibly originates from its interactions with iron/heme-containing proteins, the underlying mechanisms are still largely unclear. Since the liver plays an irreplaceable role in iron and heme metabolism in the body, it is essential to investigate how thorium affects iron and heme homeostasis in hepatocytes. In this study, we first assessed the liver injury in mice exposed to tetravalent thorium (Th(IV)) in the form of thorium nitrite via the oral route. After a two-week oral exposure, thorium accumulation and iron overload were observed in the liver, which are both closely associated with lipid peroxidation and cell death. Transcriptomics analysis revealed that ferroptosis, which has not previously been documented in cells for actinides, is the main mechanism of programmed cell death induced by Th(IV). Further mechanistic studies suggested that Th(IV) could activate the ferroptotic pathway through disrupting iron homeostasis and generating lipid peroxides. More significantly, the disorder of heme metabolism, which is crucial for maintaining intracellular iron and redox homeostasis, was found to contribute to ferroptosis in hepatocytes exposed to Th(IV). Our findings may shed light on a key mechanism of hepatoxicity in response to Th(IV) stress and provide in-depth understanding of the health risk of thorium.
中文翻译:

钍 (IV) 通过破坏口服摄入后肝脏中的铁稳态和血红素代谢引发铁死亡
钍是稀土采矿业的副产品,可用作下一代核电设施的燃料,这可能对人们的健康构成威胁。尽管已发表的文献表明钍的毒性可能源于它与含铁/含血红素的蛋白质的相互作用,但其潜在机制在很大程度上仍不清楚。由于肝脏在体内铁和血红素代谢中起着不可替代的作用,因此有必要研究钍如何影响肝细胞中铁和血红素的稳态。在这项研究中,我们首先评估了通过口服途径暴露于亚硝酸钍形式的四价钍 (Th(IV)) 的小鼠的肝损伤。经口暴露两周后,在肝脏中观察到钍积累和铁过载,这两者都与脂质过氧化和细胞死亡密切相关。转录组学分析表明,铁死亡是 Th(IV) 诱导的程序性细胞死亡的主要机制,此前未在锕系元素细胞中记录到铁死亡。进一步的机理研究表明,Th(IV) 可以通过破坏铁稳态和产生脂质过氧化物来激活铁死亡途径。更重要的是,血红素代谢紊乱对维持细胞内铁和氧化还原稳态至关重要,被发现会导致暴露于 Th(IV) 的肝细胞发生铁死亡。我们的研究结果可能阐明肝毒性响应 Th(IV) 应激的关键机制,并提供对钍健康风险的深入了解。以前在细胞中没有记录到锕系元素,这是 Th(IV) 诱导的程序性细胞死亡的主要机制。进一步的机理研究表明,Th(IV) 可以通过破坏铁稳态和产生脂质过氧化物来激活铁死亡途径。更重要的是,血红素代谢紊乱对维持细胞内铁和氧化还原稳态至关重要,被发现会导致暴露于 Th(IV) 的肝细胞发生铁死亡。我们的研究结果可能阐明肝毒性响应 Th(IV) 应激的关键机制,并提供对钍健康风险的深入了解。以前在细胞中没有记录到锕系元素,这是 Th(IV) 诱导的程序性细胞死亡的主要机制。进一步的机理研究表明,Th(IV) 可以通过破坏铁稳态和产生脂质过氧化物来激活铁死亡途径。更重要的是,血红素代谢紊乱对维持细胞内铁和氧化还原稳态至关重要,被发现会导致暴露于 Th(IV) 的肝细胞发生铁死亡。我们的研究结果可能阐明肝毒性响应 Th(IV) 应激的关键机制,并提供对钍健康风险的深入了解。进一步的机理研究表明,Th(IV) 可以通过破坏铁稳态和产生脂质过氧化物来激活铁死亡途径。更重要的是,血红素代谢紊乱对维持细胞内铁和氧化还原稳态至关重要,被发现会导致暴露于 Th(IV) 的肝细胞发生铁死亡。我们的研究结果可能阐明肝毒性响应 Th(IV) 应激的关键机制,并提供对钍健康风险的深入了解。进一步的机理研究表明,Th(IV) 可以通过破坏铁稳态和产生脂质过氧化物来激活铁死亡途径。更重要的是,血红素代谢紊乱对维持细胞内铁和氧化还原稳态至关重要,被发现会导致暴露于 Th(IV) 的肝细胞发生铁死亡。我们的研究结果可能阐明肝毒性响应 Th(IV) 应激的关键机制,并提供对钍健康风险的深入了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号