当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid Enantiomeric Excess Measurements of Enantioisotopomers by Molecular Rotational Resonance Spectroscopy
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-03-17 , DOI: 10.1021/acs.oprd.3c00028 Reilly E Sonstrom 1 , Zoua Pa Vang 2 , Haley N Scolati 3 , Justin L Neill 1 , Brooks H Pate 3 , Joseph R Clark 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-03-17 , DOI: 10.1021/acs.oprd.3c00028 Reilly E Sonstrom 1 , Zoua Pa Vang 2 , Haley N Scolati 3 , Justin L Neill 1 , Brooks H Pate 3 , Joseph R Clark 2
Affiliation
|
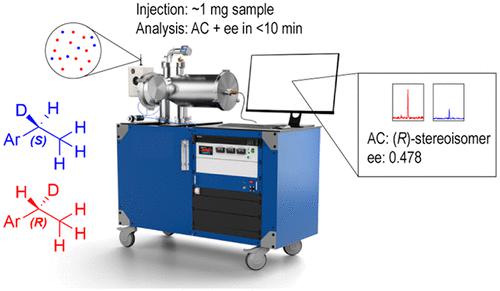
Recent work in drug discovery has shown that selectively deuterated small molecules can improve the safety and efficacy for active pharmaceutical ingredients. The advantages derive from changes in metabolism resulting from the kinetic isotope effect when deuterium is substituted for a hydrogen atom at a structural position where rate limiting C–H bond breaking occurs. This application has pushed the development of precision deuteration strategies in synthetic chemistry that can install deuterium atoms with high regioselectivity and with stereocontrol. Copper-catalyzed alkene transfer hydrodeuteration chemistry has recently been shown to have high stereoselectivity for deuteration at the metabolically important benzyl C–H position. In this case, stereocontrol results in the creation of enantioisotopomers─molecules that are chiral solely by virtue of the deuterium substitution─and chiral analysis techniques are needed to assess the reaction selectivity. It was recently shown that chiral tag molecular rotational resonance (MRR) spectroscopy provides a routine way to measure the enantiomeric excess and establish the absolute configuration of enantioisotopomers. High-throughput implementations of chiral tag MRR spectroscopy are needed to support optimization of the chemical synthesis. A measurement methodology for high-throughput chiral analysis is demonstrated in this work. The high-throughput ee measurements are performed using cavity-enhanced MRR spectroscopy, which reduces measurement times and sample consumption by more than an order-of-magnitude compared to the previous enantioisotopomer analysis using a broadband MRR spectrometer. It is also shown that transitions for monitoring the enantiomers can be selected from a broadband rotational spectrum without the need for spectroscopic analysis. The general applicability of chiral tag MRR spectroscopy is illustrated by performing chiral analysis on six enantioisotopomer reaction products using a single molecule as the tag for chiral discrimination.
中文翻译:

通过分子旋转共振光谱法快速测量对映异构体过量
最近的药物发现工作表明,选择性氘化小分子可以提高活性药物成分的安全性和有效性。这些优点源自当氘在发生限速 C-H 键断裂的结构位置上取代氢原子时,动力学同位素效应导致的代谢变化。这一应用推动了合成化学中精密氘化策略的发展,该策略可以以高区域选择性和立体控制安装氘原子。铜催化的烯烃转移加氢氘化化学最近被证明对代谢重要的苄基 C-H 位点的氘化具有高立体选择性。在这种情况下,立体控制导致对映同位素异构体的产生(仅通过氘取代而成为手性的分子),并且需要手性分析技术来评估反应选择性。最近表明,手性标签分子旋转共振(MRR)光谱提供了一种测量对映体过量并建立对映体同位素绝对构型的常规方法。需要高通量实施手性标签 MRR 光谱来支持化学合成的优化。这项工作展示了高通量手性分析的测量方法。高通量ee测量是使用腔增强MRR光谱进行的,与之前使用宽带MRR光谱仪的对映同位素分析相比,这将测量时间和样品消耗减少了一个数量级以上。 还表明,可以从宽带旋转光谱中选择用于监测对映体的转变,而不需要光谱分析。通过使用单分子作为手性判别标签对六种对映同位素反应产物进行手性分析,说明了手性标签 MRR 光谱的一般适用性。
更新日期:2023-03-17
中文翻译:

通过分子旋转共振光谱法快速测量对映异构体过量
最近的药物发现工作表明,选择性氘化小分子可以提高活性药物成分的安全性和有效性。这些优点源自当氘在发生限速 C-H 键断裂的结构位置上取代氢原子时,动力学同位素效应导致的代谢变化。这一应用推动了合成化学中精密氘化策略的发展,该策略可以以高区域选择性和立体控制安装氘原子。铜催化的烯烃转移加氢氘化化学最近被证明对代谢重要的苄基 C-H 位点的氘化具有高立体选择性。在这种情况下,立体控制导致对映同位素异构体的产生(仅通过氘取代而成为手性的分子),并且需要手性分析技术来评估反应选择性。最近表明,手性标签分子旋转共振(MRR)光谱提供了一种测量对映体过量并建立对映体同位素绝对构型的常规方法。需要高通量实施手性标签 MRR 光谱来支持化学合成的优化。这项工作展示了高通量手性分析的测量方法。高通量ee测量是使用腔增强MRR光谱进行的,与之前使用宽带MRR光谱仪的对映同位素分析相比,这将测量时间和样品消耗减少了一个数量级以上。 还表明,可以从宽带旋转光谱中选择用于监测对映体的转变,而不需要光谱分析。通过使用单分子作为手性判别标签对六种对映同位素反应产物进行手性分析,说明了手性标签 MRR 光谱的一般适用性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号