Cell ( IF 45.5 ) Pub Date : 2023-03-16 , DOI: 10.1016/j.cell.2023.02.023 Sima Khazaei 1 , Carol C L Chen 1 , Augusto Faria Andrade 1 , Nisha Kabir 1 , Pariya Azarafshar 1 , Shahir M Morcos 2 , Josiane Alves França 3 , Mariana Lopes 4 , Peder J Lund 4 , Geoffroy Danieau 5 , Samantha Worme 6 , Lata Adnani 7 , Nadine Nzirorera 1 , Xiao Chen 8 , Gayathri Yogarajah 9 , Caterina Russo 10 , Michele Zeinieh 1 , Cassandra J Wong 11 , Laura Bryant 12 , Steven Hébert 13 , Bethany Tong 14 , Tianna S Sihota 1 , Damien Faury 7 , Evan Puligandla 15 , Wajih Jawhar 16 , Veronica Sandy 7 , Mitra Cowan 17 , Emily M Nakada 7 , Loydie A Jerome-Majewska 18 , Benjamin Ellezam 19 , Carolina Cavalieri Gomes 3 , Jonas Denecke 20 , Davor Lessel 21 , Marie T McDonald 22 , Carolyn E Pizoli 23 , Kathryn Taylor 22 , Benjamin T Cocanougher 24 , Elizabeth J Bhoj 25 , Anne-Claude Gingras 26 , Benjamin A Garcia 4 , Chao Lu 27 , Eric I Campos 2 , Claudia L Kleinman 13 , Livia Garzia 5 , Nada Jabado 28
|
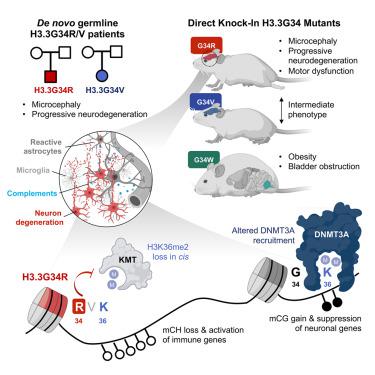
Germline histone H3.3 amino acid substitutions, including H3.3G34R/V, cause severe neurodevelopmental syndromes. To understand how these mutations impact brain development, we generated H3.3G34R/V/W knock-in mice and identified strikingly distinct developmental defects for each mutation. H3.3G34R-mutants exhibited progressive microcephaly and neurodegeneration, with abnormal accumulation of disease-associated microglia and concurrent neuronal depletion. G34R severely decreased H3K36me2 on the mutant H3.3 tail, impairing recruitment of DNA methyltransferase DNMT3A and its redistribution on chromatin. These changes were concurrent with sustained expression of complement and other innate immune genes possibly through loss of non-CG (CH) methylation and silencing of neuronal gene promoters through aberrant CG methylation. Complement expression in G34R brains may lead to neuroinflammation possibly accounting for progressive neurodegeneration. Our study reveals that H3.3G34-substitutions have differential impact on the epigenome, which underlie the diverse phenotypes observed, and uncovers potential roles for H3K36me2 and DNMT3A-dependent CH-methylation in modulating synaptic pruning and neuroinflammation in post-natal brains.
中文翻译:

H3.3G34 中的单一替换改变 DNMT3A 募集,导致进行性神经变性
种系组蛋白 H3.3 氨基酸取代(包括 H3.3G34R/V)会导致严重的神经发育综合征。为了了解这些突变如何影响大脑发育,我们培育了 H3.3G34R/V/W 敲入小鼠,并确定了每种突变的显着不同的发育缺陷。 H3.3G34R 突变体表现出进行性小头畸形和神经变性,伴有疾病相关小胶质细胞的异常积累和并发的神经元耗竭。 G34R 严重降低突变体 H3.3 尾部的 H3K36me2,损害 DNA 甲基转移酶 DNMT3A 的募集及其在染色质上的重新分配。这些变化与补体和其他先天免疫基因的持续表达同时发生,可能是由于非 CG (CH) 甲基化的丧失以及通过异常 CG 甲基化导致的神经元基因启动子的沉默。 G34R 大脑中的补体表达可能导致神经炎症,这可能是进行性神经变性的原因。我们的研究表明,H3.3G34 取代对表观基因组有不同的影响,表观基因组是观察到的不同表型的基础,并揭示了 H3K36me2 和 DNMT3A 依赖性 CH 甲基化在调节产后大脑突触修剪和神经炎症中的潜在作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号