当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biocatalytic Asymmetric Reduction of a Sterically Hindered α-Bromo Ketone for the Synthesis of Key Intermediates of Olodaterol
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-03-15 , DOI: 10.1021/acs.oprd.2c00371 Yahui Feng 1, 2 , Zihong Zhou 1, 2, 3 , Shuming Wu 1, 2 , Wei Lin 2 , Songquan Lu 2 , Xiaolei Pang 2 , Ke Xia 2 , Fang He 2 , Qin Zhang 2, 4 , Hu Yang 2 , Zhongqing Wang 1, 2, 5
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-03-15 , DOI: 10.1021/acs.oprd.2c00371 Yahui Feng 1, 2 , Zihong Zhou 1, 2, 3 , Shuming Wu 1, 2 , Wei Lin 2 , Songquan Lu 2 , Xiaolei Pang 2 , Ke Xia 2 , Fang He 2 , Qin Zhang 2, 4 , Hu Yang 2 , Zhongqing Wang 1, 2, 5
Affiliation
|
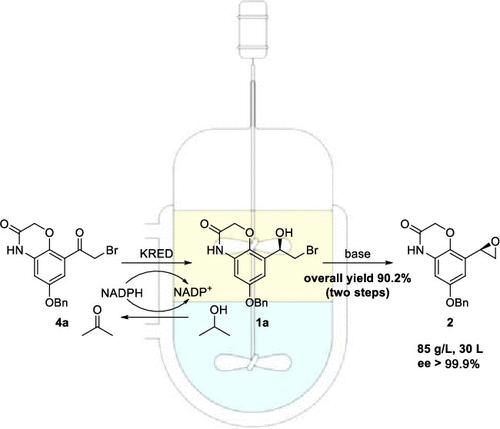
An efficient and selective biocatalytic process was developed for the reduction of highly bulky and hydrophobic α-bromo ketone 4a using ketoreductase from Lactobacillus kefiri. A fter the initial identification of this selective reductase, reaction conversion was increased from 25.7 to 94.3% and the substrate concentration increased from 5 to 85 g/L via detailed optimization. Through intramolecular epoxidation, the obtained α-bromohydrin 1a was transformed into epoxide 2 as the final product with >99.9% ee, 98.5% HPLC purity, and 90.2% yield over two steps. Eventually, a scale-up demonstration of the telescoped process was successfully performed at a 30 L scale showing excellent reproducibility and consistency, guaranteeing the obtainment of olodaterol with good quality. This biocatalytic process was further demonstrated as environmentally benign by a 50% reduction in process mass intensity (PMI) when compared to the reported (-)-DIP-Cl process.
中文翻译:

空间位阻 α-溴酮的生物催化不对称还原合成关键中间体奥达特罗
使用来自凯菲里乳杆菌的酮还原酶,开发了一种有效且选择性的生物催化过程,用于减少体积庞大且疏水的 α-溴代酮4a。在初步鉴定出这种选择性还原酶后,通过详细优化,反应转化率从 25.7% 提高到 94.3%,底物浓度从 5 g/L 提高到 85 g/L。通过分子内环氧化反应,将得到的α-溴醇1a转化为环氧化物2作为最终产品,具有 >99.9% ee、98.5% HPLC 纯度和 90.2% 两步收率。最终,在 30 L 规模上成功进行了伸缩过程的放大演示,显示出出色的重现性和一致性,保证了获得高质量的奥达特罗。与报道的 (-)-DIP-Cl 工艺相比,该生物催化工艺通过工艺质量强度 (PMI) 降低 50% 进一步证明对环境无害。
更新日期:2023-03-15
中文翻译:

空间位阻 α-溴酮的生物催化不对称还原合成关键中间体奥达特罗
使用来自凯菲里乳杆菌的酮还原酶,开发了一种有效且选择性的生物催化过程,用于减少体积庞大且疏水的 α-溴代酮4a。在初步鉴定出这种选择性还原酶后,通过详细优化,反应转化率从 25.7% 提高到 94.3%,底物浓度从 5 g/L 提高到 85 g/L。通过分子内环氧化反应,将得到的α-溴醇1a转化为环氧化物2作为最终产品,具有 >99.9% ee、98.5% HPLC 纯度和 90.2% 两步收率。最终,在 30 L 规模上成功进行了伸缩过程的放大演示,显示出出色的重现性和一致性,保证了获得高质量的奥达特罗。与报道的 (-)-DIP-Cl 工艺相比,该生物催化工艺通过工艺质量强度 (PMI) 降低 50% 进一步证明对环境无害。











































 京公网安备 11010802027423号
京公网安备 11010802027423号