当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Base-mediated chalcogenoaminative annulation of 2-alkynylanilines for direct access to 3-sulfenyl/selenyl-1H-indoles
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2023-03-15 , DOI: 10.1039/d3ob00279a Wei-Ching Chen, Rekha Bai, Wan-Lin Cheng, Chun-Yu Peng, Daggula Mallikarjuna Reddy, Satpal Singh Badsara, Chin-Fa Lee
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2023-03-15 , DOI: 10.1039/d3ob00279a Wei-Ching Chen, Rekha Bai, Wan-Lin Cheng, Chun-Yu Peng, Daggula Mallikarjuna Reddy, Satpal Singh Badsara, Chin-Fa Lee
|
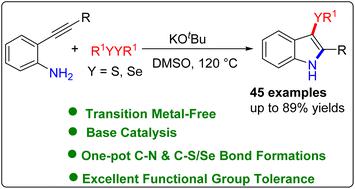
An efficient and transition metal-free synthesis of 3-sulfenyl/selenyl-1H-indoles via a base-assisted chalcogenoaminative annulation of 2-alkynyl aniline with disulfides/diselenides is described. A series of 2-alkynylanilines were found compatible with dichalcogenides in this transformation providing 3-sulfenyl/selenyl-1H-indoles in good to excellent yields. The presented methodology has the advantages of easily available raw materials, functional group tolerance, and a wide range of substrates that provide access to 3-sulfenylindoles and 3-selenylindoles.
中文翻译:

碱基介导的 2-炔基苯胺的硫族氨基化环化可直接获得 3-硫基/硒基-1H-吲哚
描述了通过2-炔基苯胺与二硫化物/二硒化物的碱辅助硫族胺化环化,高效且无过渡金属的 3-sulfenyl/selenyl-1 H - indoles合成。发现一系列 2-炔基苯胺与二硫化物在该转化中相容,可提供 3-sulfenyl/selenyl-1 H -indoles,产率良好至极佳。所提出的方法具有易于获得的原材料、官能团耐受性以及提供获取 3-sulfenylindoles 和 3-selenylindoles 的广泛底物的优点。
更新日期:2023-03-15
中文翻译:

碱基介导的 2-炔基苯胺的硫族氨基化环化可直接获得 3-硫基/硒基-1H-吲哚
描述了通过2-炔基苯胺与二硫化物/二硒化物的碱辅助硫族胺化环化,高效且无过渡金属的 3-sulfenyl/selenyl-1 H - indoles合成。发现一系列 2-炔基苯胺与二硫化物在该转化中相容,可提供 3-sulfenyl/selenyl-1 H -indoles,产率良好至极佳。所提出的方法具有易于获得的原材料、官能团耐受性以及提供获取 3-sulfenylindoles 和 3-selenylindoles 的广泛底物的优点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号