当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantio- and diastereodivergent synthesis of fused indolizines enabled by synergistic Cu/Ir catalysis
Chemical Science ( IF 7.6 ) Pub Date : 2023-03-14 , DOI: 10.1039/d3sc00118k Bing-Ke Zhu 1, 2 , Hui Xu 3 , Lu Xiao 1 , Xin Chang 1 , Liang Wei 1 , Huailong Teng 4 , Yanfeng Dang 3 , Xiu-Qin Dong 1 , Chun-Jiang Wang 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2023-03-14 , DOI: 10.1039/d3sc00118k Bing-Ke Zhu 1, 2 , Hui Xu 3 , Lu Xiao 1 , Xin Chang 1 , Liang Wei 1 , Huailong Teng 4 , Yanfeng Dang 3 , Xiu-Qin Dong 1 , Chun-Jiang Wang 1, 2
Affiliation
|
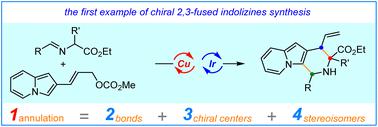
Highly diastereo-/enantioselective assembly of 2,3-fused indolizine derivatives could be easily available through a cascade allylation/Friedel–Crafts type reaction enabled by a synergistic Cu/Ir catalysis. This designed protocol provides an unprecedented and facile route to enantioenriched indolizines bearing three stereogenic centers in moderate to high yields with excellent stereoselective control, which also featured broad substrate generality. Remarkably, four stereoisomers of the 2,3-fused indolizine products could be efficiently constructed in a predictable manner through the pairwise combination of copper and iridium catalysts. The synthetic utility of this method was readily elaborated by a gram-scale reaction, and synthetic transformations to other important chiral indolizine derivatives. Quantum mechanical explorations constructed a plausible synergetic catalytic cycle, revealed the origins of stereodivergence, and rationalized the protonation-stimulated stereoselective Friedel–Crafts type cyclization to form the indolizine products.
中文翻译:

通过协同 Cu/Ir 催化实现稠合中氮嗪的对映体和非对映体合成
通过由协同 Cu/Ir 催化实现的级联烯丙基化/Friedel-Crafts 型反应,可以很容易地获得 2,3-稠合中吲嗪衍生物的高度非对映/对映选择性组装。这种设计的方案提供了一种前所未有的简便途径,可以以中等到高的收率获得具有三个立体异构中心的对映体富集吲嗪,具有出色的立体选择性控制,还具有广泛的底物通用性。值得注意的是,通过铜和铱催化剂的成对组合,可以以可预测的方式有效地构建 2,3-稠合中吲嗪产物的四种立体异构体。这种方法的合成效用很容易通过克级反应和其他重要的手性中吲嗪衍生物的合成转化得到阐述。
更新日期:2023-03-14
中文翻译:

通过协同 Cu/Ir 催化实现稠合中氮嗪的对映体和非对映体合成
通过由协同 Cu/Ir 催化实现的级联烯丙基化/Friedel-Crafts 型反应,可以很容易地获得 2,3-稠合中吲嗪衍生物的高度非对映/对映选择性组装。这种设计的方案提供了一种前所未有的简便途径,可以以中等到高的收率获得具有三个立体异构中心的对映体富集吲嗪,具有出色的立体选择性控制,还具有广泛的底物通用性。值得注意的是,通过铜和铱催化剂的成对组合,可以以可预测的方式有效地构建 2,3-稠合中吲嗪产物的四种立体异构体。这种方法的合成效用很容易通过克级反应和其他重要的手性中吲嗪衍生物的合成转化得到阐述。









































 京公网安备 11010802027423号
京公网安备 11010802027423号