Structure ( IF 4.4 ) Pub Date : 2023-03-13 , DOI: 10.1016/j.str.2023.02.010 Amy E Fraley 1 , Maria Dell 1 , Maximilian Schmalhofer 2 , Roy A Meoded 1 , Cedric Bergande 1 , Michael Groll 2 , Jörn Piel 1
|
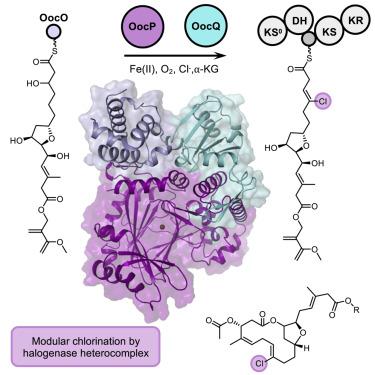
Bacterial modular polyketide synthases (PKSs) generate diverse, complex and bioactive natural products that are constructed mainly based on principles of fatty acid biosynthesis. The cytotoxic oocydin-type polyketides contain a vinyl chloride moiety introduced during polyketide chain elongation. Required for modular polyketide backbone halogenation are a non-heme iron and ɑ-ketoglutarate-dependent halogenase OocP and OocQ lacking characterized homologs. This work provides structural insights into these unusual PKS components and their interactions via a high-resolution X-ray crystallography structure of the heterocomplex. By mapping the protein-protein interactions and comparison with structures of similar halogenases, we illustrate the potential of this heterodimer complex as a replacement for the conserved homodimeric structure of homologous enzymes. The OocPQ protein pair has thus evolved as a means of stabilizing the halogenase and facilitating chemical transformations with great synthetic utility.
中文翻译:

参与模块化骨架卤化的聚酮合酶成分的异质复合结构
细菌模块化聚酮化合物合酶(PKS) 产生多种多样、复杂且具有生物活性的天然产物,这些天然产物主要基于脂肪酸生物合成原理构建。细胞毒性卵胞素型聚酮化合物含有在聚酮化合物链延长过程中引入的氯乙烯部分。模块化聚酮主链卤化所需是一种非血红素铁和 ɑ-酮戊二酸依赖性卤化酶 OocP 和 OocQ,缺乏特征同系物。这项工作通过异质复合物的高分辨率 X 射线晶体学结构提供了对这些不寻常的 PKS 组件及其相互作用的结构见解。通过绘制蛋白质-蛋白质相互作用图并与类似卤素酶的结构进行比较,我们说明了这种异二聚体复合物作为同源酶的保守同二聚体结构替代品的潜力。因此,OocPQ 蛋白对已发展成为稳定卤化酶和促进化学转化的一种手段,具有巨大的合成效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号