Journal of Catalysis ( IF 7.3 ) Pub Date : 2023-03-13 , DOI: 10.1016/j.jcat.2023.03.020 Chang Yao , Wenhua Li , Yueqiang Cao , Xiaohu Ge , Zhirong Yang , Gang Qian , Xinggui Zhou , Xuezhi Duan
|
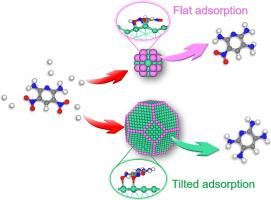
Designing cost-effective Ni-based catalysts with satisfactory performance for the hydrogenation of 2,6-diamino-3,5-dinitropyridine (DADNP) to 2,3,5,6-tetraaminopyridine (TAP) is highly essential for producing high-performance poly (pyridobisimidazole) but remains a great challenge due to the limited mechanistic and kinetics understandings. Herein, a series of differently sized Ni catalysts but similar electronic properties are synthesized to explore the size-dependent kinetics behaviors of the hydrogenation of DADNP via two competitive routes. The results unravel that the hydrogenation of DADNP to TAP via the route 2, i.e., the formation of TAP from the direct hydrogenation of DADNP without being intermediated by the 2,3,6-triamino-5-nitropyridine (TANP), appears to be more prominent on larger sized Ni catalysts. Microkinetics studies based on the Langmuir-Hinshelwood-Hougen-Watson model indicate that the ratio of the TAP formation rates via these two routes, i.e., k´TAP2/k´TAP1, increases with the increasing Ni particle size, confirming the more favorable formation of TAP via the route 2 on larger sized Ni catalysts. These size-dependent kinetics behaviors are further rationalized by DFT calculations, which reveal that the simultaneous activation of both nitro groups of DADNP via flat adsorption is more likely to occur on the larger size Ni catalysts than that on the smaller sized ones.
中文翻译:

Ni 催化剂上 2,6-二氨基-3,5-二硝基吡啶氢化的结构敏感性的机理和动力学见解
设计具有令人满意的 2,6-二氨基-3,5-二硝基吡啶 (DADNP) 加氢成 2,3,5,6-四氨基吡啶 (TAP) 性能的经济高效的镍基催化剂对于生产高性能催化剂非常重要聚(吡啶并双咪唑),但由于对机理和动力学的理解有限,这仍然是一个巨大的挑战。在此,合成了一系列不同尺寸但具有相似电子特性的 Ni 催化剂,以通过两条竞争路线探索 DADNP 氢化的尺寸依赖性动力学行为。结果表明,通过路线 2 将 DADNP 氢化为 TAP,即通过 DADNP 的直接氢化形成 TAP,而无需通过 2,3,6-三氨基-5-硝基吡啶 (TANP) 进行中介,似乎是在较大尺寸的 Ni 催化剂上更为突出。k' TAP2 / k' TAP1随着 Ni 粒径的增加而增加,证实了在较大尺寸的 Ni 催化剂上通过路线 2 更有利地形成 TAP。这些尺寸相关的动力学行为通过 DFT 计算进一步合理化,这表明通过平面吸附同时激活 DADNP 的两个硝基基团更可能发生在较大尺寸的 Ni 催化剂上而不是较小尺寸的 Ni 催化剂上。

































 京公网安备 11010802027423号
京公网安备 11010802027423号