Waste Management ( IF 7.1 ) Pub Date : 2023-03-12 , DOI: 10.1016/j.wasman.2023.03.007 Yu Chen 1 , Lifeng Tian 2 , Tingting Liu 3 , Zewei Liu 3 , Zechun Huang 3 , Haoyue Yang 3 , Lu Tian 3 , Qifei Huang 3 , Weishi Li 3 , Yanjiao Gao 4 , Zhao Zhang 4
|
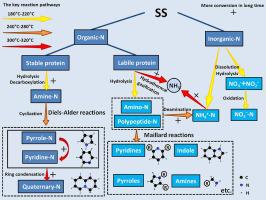
Hydrothermal carbonization (HTC) is an effective means of energizing high-water-content biomass that can be used to convert sewage sludge (SS) into hydrochar and reduce nitrogen content. To further reduce the emission of NOx during the combustion of hydrochar and seek proper disposal method of liquid product, the mechanism of nitrogen conversion was studied in the range of 180–320 °C and 30–90 min. At 180–220 °C, 42.15–52.91% of the nitrogen in SS was transferred to liquid by hydrolysis of proteins and inorganic salts. At 240–280 °C, the nitrogen in hydrochar was mainly in the form of heterocyclic -N (quaternary-N, pyrrole-N, and pyridine-N). The concentration of NH4+-N increased from 6.82 mg/L (180 °C) to 26.58 mg/L (280 °C) due to the enhancement of the deamination reaction. At 300–320 °C, pyrrole-N (from 15.92% to 9.38%) and pyridine-N (from 5.52% to 3.73%) in the hydrochar were converted to the more stable quaternary-N (from 0.31% to 4.28%). Meanwhile, the NH4+-N and amino-N in the liquid decomposed into NH3. Prolonging the carbonization time promoted the hydrolysis of proteins, the conversion of heterocyclic -N, and the production of NH3. Under optimal reaction conditions (280 °C and 60 min), the nitrogen in the SS is converted to stable forms and the energy balance meets the requirements of circular-economy. The results show that temperature determines the nitrogen form and the carbonization time affects the nitrogen distribution. So HTC has the potential to reduce NOx emissions from SS energy utilization processes.
中文翻译:

污水污泥水热炭化氮的形态和转化-温度和炭化时间的影响
水热碳化 (HTC) 是为高含水量生物质提供能量的有效手段,可用于将污水污泥 (SS) 转化为水炭并降低氮含量。为进一步减少水力炭燃烧过程中NOx的排放,寻求液体产物的合适处理方法,研究了180~320℃、30~90 min的氮转化机理。在 180–220 °C 下,SS 中 42.15–52.91% 的氮通过蛋白质和无机盐的水解转移到液体中。在 240–280 °C 时,水炭中的氮主要以杂环-N(季-N、吡咯-N 和吡啶-N)的形式存在。NH 4 +浓度由于脱氨反应的增强,-N 从 6.82 mg/L (180 °C) 增加到 26.58 mg/L (280 °C)。在 300–320 °C 下,水炭中的吡咯-N(从 15.92% 到 9.38%)和吡啶-N(从 5.52% 到 3.73%)转化为更稳定的季氮(从 0.31% 到 4.28%) . 同时,液体中的NH 4 + -N和氨基-N分解为NH 3。延长碳化时间促进蛋白质的水解、杂环-N的转化和NH 3的产生. 在最佳反应条件下(280 °C 和 60 分钟),SS 中的氮转化为稳定形式,能量平衡满足循环经济的要求。结果表明,温度决定了氮的形态,碳化时间影响了氮的分布。因此,HTC 有可能减少 SS 能源利用过程中的 NOx排放。











































 京公网安备 11010802027423号
京公网安备 11010802027423号