当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Scalable Asymmetric Process for the Synthesis of GLYT1 Inhibitor BI 425809 (Iclepertin)
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-03-03 , DOI: 10.1021/acs.oprd.2c00373 Rogelio Frutos 1 , Thomas G. Tampone 1 , Jason Mulder 1 , Joe Gao 1 , Joshua D. Sieber 1 , Sonia Rodriguez 1 , Nizar Haddad 1 , Katrin Baer 1 , Jack Brown 1 , Bing-Shiou Yang 1 , Riccardo Giovannini 1 , Jinhua J. Song 1 , Nelu Grinberg 1 , Heewon Lee 1 , Chris H. Senanayake 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-03-03 , DOI: 10.1021/acs.oprd.2c00373 Rogelio Frutos 1 , Thomas G. Tampone 1 , Jason Mulder 1 , Joe Gao 1 , Joshua D. Sieber 1 , Sonia Rodriguez 1 , Nizar Haddad 1 , Katrin Baer 1 , Jack Brown 1 , Bing-Shiou Yang 1 , Riccardo Giovannini 1 , Jinhua J. Song 1 , Nelu Grinberg 1 , Heewon Lee 1 , Chris H. Senanayake 1
Affiliation
|
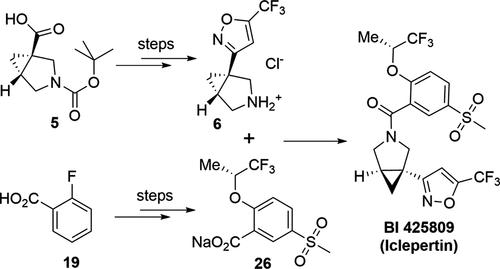
A robust and scalable synthesis process for BI 425809 (Iclepertin), a GLYT1 inhibitor with potential therapeutic properties for the treatment of central nervous system disorders, was developed and implemented on a multikilogram scale. Key aspects of the process include the efficient asymmetric synthesis of intermediate 3-((1R,5R)-3-azabicyclo[3.1.0]hexan-1-yl)-5-(trifluoromethyl)isoxazole·HCl from raw materials readily available in bulk and the synthesis of (R)-5-(methylsulfonyl)-2-((1,1,1-trifluoropropan-2-yl)oxy)benzoic acid through a novel Rh-catalyzed asymmetric hydrogenation.
中文翻译:

开发用于合成 GLYT1 抑制剂 BI 425809 (Iclepertin) 的可扩展不对称工艺
BI 425809 ( Iclepertin )是一种稳健且可扩展的合成工艺,它是一种 GLYT1 抑制剂,具有治疗中枢神经系统疾病的潜在治疗特性,已开发并以数千克规模实施。该工艺的关键方面包括从原料中高效不对称合成中间体 3-((1 R ,5 R )-3-氮杂双环[3.1.0]hexan-1-yl)-5-(三氟甲基)异恶唑·HCl通过新型 Rh 催化的不对称氢化,可大量获得并合成 ( R )-5-(甲基磺酰基)-2-((1,1,1-三氟丙-2-基)氧基)苯甲酸。
更新日期:2023-03-03
中文翻译:

开发用于合成 GLYT1 抑制剂 BI 425809 (Iclepertin) 的可扩展不对称工艺
BI 425809 ( Iclepertin )是一种稳健且可扩展的合成工艺,它是一种 GLYT1 抑制剂,具有治疗中枢神经系统疾病的潜在治疗特性,已开发并以数千克规模实施。该工艺的关键方面包括从原料中高效不对称合成中间体 3-((1 R ,5 R )-3-氮杂双环[3.1.0]hexan-1-yl)-5-(三氟甲基)异恶唑·HCl通过新型 Rh 催化的不对称氢化,可大量获得并合成 ( R )-5-(甲基磺酰基)-2-((1,1,1-三氟丙-2-基)氧基)苯甲酸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号