Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amplification-Free, High-Throughput Nanoplasmonic Quantification of Circulating MicroRNAs in Unprocessed Plasma Microsamples for Earlier Pancreatic Cancer Detection
ACS Sensors ( IF 8.2 ) Pub Date : 2023-02-28 , DOI: 10.1021/acssensors.2c02105 Adrianna N Masterson 1 , Nayela N Chowdhury 2, 3 , Yue Fang 4 , Michele T Yip-Schneider 5 , Sumon Hati 1 , Prashant Gupta 6 , Sha Cao 4 , Huangbing Wu 5 , C Max Schmidt 3, 5 , Melissa L Fishel 2, 3, 7 , Rajesh Sardar 1, 3
ACS Sensors ( IF 8.2 ) Pub Date : 2023-02-28 , DOI: 10.1021/acssensors.2c02105 Adrianna N Masterson 1 , Nayela N Chowdhury 2, 3 , Yue Fang 4 , Michele T Yip-Schneider 5 , Sumon Hati 1 , Prashant Gupta 6 , Sha Cao 4 , Huangbing Wu 5 , C Max Schmidt 3, 5 , Melissa L Fishel 2, 3, 7 , Rajesh Sardar 1, 3
Affiliation
|
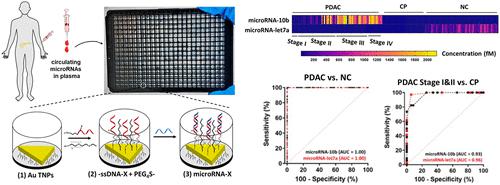
Pancreatic ductal adenocarcinoma (PDAC) is a deadly malignancy that is often detected at an advanced stage. Earlier diagnosis of PDAC is key to reducing mortality. Circulating biomarkers such as microRNAs are gaining interest, but existing technologies require large sample volumes, amplification steps, extensive biofluid processing, lack sensitivity, and are low-throughput. Here, we present an advanced nanoplasmonic sensor for the highly sensitive, amplification-free detection and quantification of microRNAs (microRNA-10b, microRNA-let7a) from unprocessed plasma microsamples. The sensor construct utilizes uniquely designed −ssDNA receptors attached to gold triangular nanoprisms, which display unique localized surface plasmon resonance (LSPR) properties, in a multiwell plate format. The formation of −ssDNA/microRNA duplex controls the nanostructure–biomolecule interfacial electronic interactions to promote the charge transfer/exciton delocalization processes and enhance the LSPR responses to achieve attomolar (10–18 M) limit of detection (LOD) in human plasma. This improve LOD allows the fabrication of a high-throughput assay in a 384-well plate format. The performance of nanoplasmonic sensors for microRNA detection was further assessed by comparing with the qRT-PCR assay of 15 PDAC patient plasma samples that shows a positive correlation between these two assays with the Pearson correlation coefficient value >0.86. Evaluation of >170 clinical samples reveals that oncogenic microRNA-10b and tumor suppressor microRNA-let7a levels can individually differentiate PDAC from chronic pancreatitis and normal controls with >94% sensitivity and >94% specificity at a 95% confidence interval (CI). Furthermore, combining both oncogenic and tumor suppressor microRNA levels significantly improves differentiation of PDAC stages I and II versus III and IV with >91% and 87% sensitivity and specificity, respectively, in comparison to the sensitivity and specificity values for individual microRNAs. Moreover, we show that the level of microRNAs varies substantially in pre- and post-surgery PDAC patients (n = 75). Taken together, this ultrasensitive nanoplasmonic sensor with excellent sensitivity and specificity is capable of assaying multiple biomarkers simultaneously and may facilitate early detection of PDAC to improve patient care.
中文翻译:

对未处理血浆微样品中循环 MicroRNA 进行无扩增、高通量纳米等离子体定量,用于早期胰腺癌检测
胰腺导管腺癌(PDAC)是一种致命的恶性肿瘤,通常在晚期才被发现。 PDAC 的早期诊断是降低死亡率的关键。 microRNA 等循环生物标志物越来越受到人们的关注,但现有技术需要大量样本、扩增步骤、大量的生物流体处理、缺乏灵敏度且通量低。在这里,我们提出了一种先进的纳米等离子体传感器,用于对未处理的血浆微样品中的 microRNA(microRNA-10b、microRNA-let7a)进行高灵敏度、无扩增检测和定量。该传感器结构利用独特设计的-ssDNA受体附着在金三角纳米棱柱上,以多孔板的形式显示出独特的局域表面等离子共振(LSPR)特性。 −ssDNA/microRNA 双链体的形成控制纳米结构-生物分子界面电子相互作用,以促进电荷转移/激子离域过程并增强局域表面等离子体共振响应,从而在人血浆中实现阿托摩尔 (10 –18 M) 的检测限 (LOD)。这种改进的 LOD 允许以 384 孔板形式进行高通量测定。通过与 15 个 PDAC 患者血浆样本的 qRT-PCR 检测进行比较,进一步评估了纳米等离子体传感器用于 microRNA 检测的性能,结果表明这两种检测之间呈正相关,Pearson 相关系数值 >0.86。对超过 170 个临床样本的评估表明,致癌 microRNA-10b 和肿瘤抑制 microRNA-let7a 水平可以单独区分 PDAC 与慢性胰腺炎和正常对照,灵敏度 >94%,特异性 >94%,置信区间 (CI) 为 95%。 此外,与单个 microRNA 的敏感性和特异性值相比,结合致癌和肿瘤抑制 microRNA 水平可显着改善 PDAC I 期和 II 期与 III 期和 IV 期的分化,灵敏度和特异性分别 >91% 和 87%。此外,我们发现 PDAC 患者术前和术后的 microRNA 水平差异很大( n = 75)。总而言之,这种超灵敏的纳米等离子体传感器具有出色的灵敏度和特异性,能够同时检测多种生物标志物,并可能有助于 PDAC 的早期检测,从而改善患者护理。
更新日期:2023-02-28
中文翻译:

对未处理血浆微样品中循环 MicroRNA 进行无扩增、高通量纳米等离子体定量,用于早期胰腺癌检测
胰腺导管腺癌(PDAC)是一种致命的恶性肿瘤,通常在晚期才被发现。 PDAC 的早期诊断是降低死亡率的关键。 microRNA 等循环生物标志物越来越受到人们的关注,但现有技术需要大量样本、扩增步骤、大量的生物流体处理、缺乏灵敏度且通量低。在这里,我们提出了一种先进的纳米等离子体传感器,用于对未处理的血浆微样品中的 microRNA(microRNA-10b、microRNA-let7a)进行高灵敏度、无扩增检测和定量。该传感器结构利用独特设计的-ssDNA受体附着在金三角纳米棱柱上,以多孔板的形式显示出独特的局域表面等离子共振(LSPR)特性。 −ssDNA/microRNA 双链体的形成控制纳米结构-生物分子界面电子相互作用,以促进电荷转移/激子离域过程并增强局域表面等离子体共振响应,从而在人血浆中实现阿托摩尔 (10 –18 M) 的检测限 (LOD)。这种改进的 LOD 允许以 384 孔板形式进行高通量测定。通过与 15 个 PDAC 患者血浆样本的 qRT-PCR 检测进行比较,进一步评估了纳米等离子体传感器用于 microRNA 检测的性能,结果表明这两种检测之间呈正相关,Pearson 相关系数值 >0.86。对超过 170 个临床样本的评估表明,致癌 microRNA-10b 和肿瘤抑制 microRNA-let7a 水平可以单独区分 PDAC 与慢性胰腺炎和正常对照,灵敏度 >94%,特异性 >94%,置信区间 (CI) 为 95%。 此外,与单个 microRNA 的敏感性和特异性值相比,结合致癌和肿瘤抑制 microRNA 水平可显着改善 PDAC I 期和 II 期与 III 期和 IV 期的分化,灵敏度和特异性分别 >91% 和 87%。此外,我们发现 PDAC 患者术前和术后的 microRNA 水平差异很大( n = 75)。总而言之,这种超灵敏的纳米等离子体传感器具有出色的灵敏度和特异性,能够同时检测多种生物标志物,并可能有助于 PDAC 的早期检测,从而改善患者护理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号