当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of biologically active [1,5]diazocino[2,1-b]quinazolinones through [4 + 4] cycloaddition of 2-alkynyl quinazolinones with aza-ortho-quinone methides
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-02-28 , DOI: 10.1039/d3qo00056g Li Pang 1 , Shu-Jun Fang 1 , Pei-Sen Zou 1 , Wang Wang 1 , Jun-Cheng Su 1 , Xiao-Qing Liu 1 , Cheng-Xue Pan 1 , Dong-Liang Mo 1 , Gui-Fa Su 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-02-28 , DOI: 10.1039/d3qo00056g Li Pang 1 , Shu-Jun Fang 1 , Pei-Sen Zou 1 , Wang Wang 1 , Jun-Cheng Su 1 , Xiao-Qing Liu 1 , Cheng-Xue Pan 1 , Dong-Liang Mo 1 , Gui-Fa Su 1
Affiliation
|
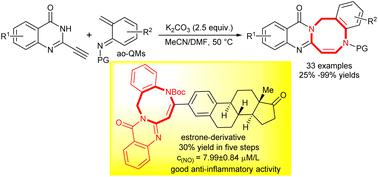
We report an atom-economical [4 + 4] cycloaddition of 2-alkynyl quinazolines with aza-ortho-quinone methides (ao-QMs) generated from 2-(bromomethyl)anilines to prepare various [1,5]diazocino[2,1-b]quinazolinones in good to excellent yields in a short reaction time and under mild reaction conditions. Moreover, an estrone-derived [1,5]diazocino[2,1-b]quinazolinone could be easily prepared in 30% total yield over five steps and biological assays reveal that the obtained [1,5]diazocino[2,1-b] quinazolinones significantly inhibited nitric oxide generation in LPS-stimulated RAW264.7 cells. The present method features transition-metal free nature, broad substrate scope, good functional group tolerance, gram-scale preparation, and simple purification operation without flash column chromatography.
中文翻译:

通过 [4 + 4] 2-炔基喹唑啉酮与氮杂邻苯醌甲基化物的环加成反应合成具有生物活性的 [1,5] 重氮并 [2,1-b] 喹唑啉酮
我们报告了 2-炔基喹唑啉与由 2-(溴甲基)苯胺生成的氮杂邻苯二酚甲基化物 (ao-QM) 的原子经济 [4 + 4] 环加成,以制备各种 [1,5] 重氮辛 [ 2,1 ] - b ]喹唑啉酮在较短的反应时间和温和的反应条件下具有良好至优异的收率。此外,一种雌酮衍生的 [1,5]diazocino[2,1- b ] 喹唑啉酮可以很容易地通过五个步骤以 30% 的总收率制备,并且生物测定表明获得的 [1,5]diazocino[2,1- b] 喹唑啉酮显着抑制 LPS 刺激的 RAW264.7 细胞中一氧化氮的产生。本方法不含过渡金属,底物适用范围广,官能团耐受性好,可实现克级制备,纯化操作简单,无需快速柱层析。
更新日期:2023-02-28
中文翻译:

通过 [4 + 4] 2-炔基喹唑啉酮与氮杂邻苯醌甲基化物的环加成反应合成具有生物活性的 [1,5] 重氮并 [2,1-b] 喹唑啉酮
我们报告了 2-炔基喹唑啉与由 2-(溴甲基)苯胺生成的氮杂邻苯二酚甲基化物 (ao-QM) 的原子经济 [4 + 4] 环加成,以制备各种 [1,5] 重氮辛 [ 2,1 ] - b ]喹唑啉酮在较短的反应时间和温和的反应条件下具有良好至优异的收率。此外,一种雌酮衍生的 [1,5]diazocino[2,1- b ] 喹唑啉酮可以很容易地通过五个步骤以 30% 的总收率制备,并且生物测定表明获得的 [1,5]diazocino[2,1- b] 喹唑啉酮显着抑制 LPS 刺激的 RAW264.7 细胞中一氧化氮的产生。本方法不含过渡金属,底物适用范围广,官能团耐受性好,可实现克级制备,纯化操作简单,无需快速柱层析。











































 京公网安备 11010802027423号
京公网安备 11010802027423号