Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereo- and Regiospecific SN2′ Reaction of MBH Adducts with Isocyanoacetates: en Route to Transition-Metal-Free α-Allylation of Isocyanoacetates
ACS Omega ( IF 4.1 ) Pub Date : 2023-02-09 , DOI: 10.1021/acsomega.2c07581 Chunsong Xie 1, 2 , Song Wu 2 , Runmei Zhang 2
ACS Omega ( IF 4.1 ) Pub Date : 2023-02-09 , DOI: 10.1021/acsomega.2c07581 Chunsong Xie 1, 2 , Song Wu 2 , Runmei Zhang 2
Affiliation
|
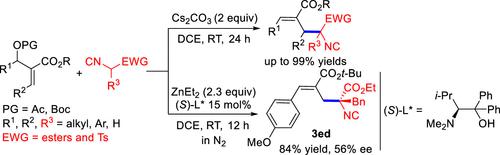
Herein, we report that under mild and transition-metal-free conditions an unprecedented and practical SN2′ reaction of Morita–Baylis–Hillman adducts with isocyanoacetates takes place in a stereo- and regiospecific manner. This reaction which tolerates a wide variety of functionalities delivers transformable α-allylated isocyanoacetates in high efficiencies. Preliminary studies on the asymmetric version of this reaction indicate that ZnEt2/chiral amino alcohol combinations are an asymmetric catalytic system for this transformation, giving an enantioenriched α-allylated isocyanoacetate with a chiral quaternary carbon in a high yield.
中文翻译:

MBH 加合物与异氰基乙酸酯的立体和区域特异性 SN2' 反应:在异氰基乙酸酯无过渡金属的 α-烯丙基化过程中
在此,我们报告说,在温和且无过渡金属的条件下,Morita-Baylis-Hillman 加合物与异氰基乙酸酯的前所未有且实用的 S N 2' 反应以立体和区域专一的方式发生。这种具有多种功能的反应可以高效地提供可转化的 α-烯丙基化异氰基乙酸酯。对该反应的不对称形式的初步研究表明,ZnEt 2 /手性氨基醇组合是该转化的不对称催化体系,可高产率地生成具有手性季碳的富含对映体的 α-烯丙基化异氰基乙酸酯。
更新日期:2023-02-09
中文翻译:

MBH 加合物与异氰基乙酸酯的立体和区域特异性 SN2' 反应:在异氰基乙酸酯无过渡金属的 α-烯丙基化过程中
在此,我们报告说,在温和且无过渡金属的条件下,Morita-Baylis-Hillman 加合物与异氰基乙酸酯的前所未有且实用的 S N 2' 反应以立体和区域专一的方式发生。这种具有多种功能的反应可以高效地提供可转化的 α-烯丙基化异氰基乙酸酯。对该反应的不对称形式的初步研究表明,ZnEt 2 /手性氨基醇组合是该转化的不对称催化体系,可高产率地生成具有手性季碳的富含对映体的 α-烯丙基化异氰基乙酸酯。



























 京公网安备 11010802027423号
京公网安备 11010802027423号