当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of Dialkyl-N-nitrosamines in Aqueous Solution: An Experimental Validation of a Conservative Predictive Model and a Comparison of the Rates of Dialkyl and Trialkylamine Nitrosation
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-02-08 , DOI: 10.1021/acs.oprd.2c00366 Ian W. Ashworth 1 , Debasis Dey 2 , Olivier Dirat 3 , Paul McDaid 4 , Daniel Lee 5 , Justin Moser 6 , Kausik K. Nanda 7
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-02-08 , DOI: 10.1021/acs.oprd.2c00366 Ian W. Ashworth 1 , Debasis Dey 2 , Olivier Dirat 3 , Paul McDaid 4 , Daniel Lee 5 , Justin Moser 6 , Kausik K. Nanda 7
Affiliation
|
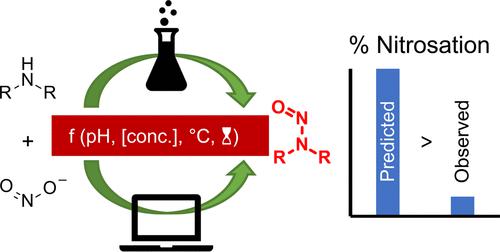
The potential for active pharmaceutical intermediates and active ingredients to contain low levels of N-nitrosamines is a topic of continued interest from industry and regulatory authorities, which has led us to generate experimental data demonstrating that the published kinetic model of dialkylamine nitrosation is conservative and may overpredict the level of N-nitrosamine formation that will actually occur. Additionally, studies comparing the nitrosation of simple trialkylamines to that of the relevant dialkylamines have demonstrated that trialkylamines do indeed undergo nitrosative dealkylation to form N-nitrosamines but at a rate that is at least 500 times lower than for the related dialkylamines.
中文翻译:

水溶液中二烷基-N-亚硝胺的形成:保守预测模型的实验验证以及二烷基胺和三烷基胺亚硝化速率的比较
活性药物中间体和活性成分含有低水平N-亚硝胺的潜力是行业和监管机构持续关注的话题,这使我们生成实验数据,证明已发表的二烷基胺亚硝化动力学模型是保守的,并且可能高估实际发生的N-亚硝胺形成水平。此外,比较简单三烷基胺与相关二烷基胺的亚硝化的研究表明,三烷基胺确实经历亚硝化脱烷基化形成N-亚硝胺,但其速率比相关二烷基胺至少低500倍。
更新日期:2023-02-08
中文翻译:

水溶液中二烷基-N-亚硝胺的形成:保守预测模型的实验验证以及二烷基胺和三烷基胺亚硝化速率的比较
活性药物中间体和活性成分含有低水平N-亚硝胺的潜力是行业和监管机构持续关注的话题,这使我们生成实验数据,证明已发表的二烷基胺亚硝化动力学模型是保守的,并且可能高估实际发生的N-亚硝胺形成水平。此外,比较简单三烷基胺与相关二烷基胺的亚硝化的研究表明,三烷基胺确实经历亚硝化脱烷基化形成N-亚硝胺,但其速率比相关二烷基胺至少低500倍。











































 京公网安备 11010802027423号
京公网安备 11010802027423号