当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphorus Overload Promotes Hepatic Lipolysis by Suppressing GSK3β-Dependent Phosphorylation of PPARα at Ser84 and Thr265 in a Freshwater Teleost
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-02-02 , DOI: 10.1021/acs.est.2c06330 Yi-Chuang Xu 1 , Kostas Pantopoulos 2 , Hua Zheng 1 , Ester Zito 3, 4 , Tao Zhao 1 , Xiao-Ying Tan 1 , Xiao-Lei Wei 1 , Yu-Feng Song 1 , Zhi Luo 1, 5
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-02-02 , DOI: 10.1021/acs.est.2c06330 Yi-Chuang Xu 1 , Kostas Pantopoulos 2 , Hua Zheng 1 , Ester Zito 3, 4 , Tao Zhao 1 , Xiao-Ying Tan 1 , Xiao-Lei Wei 1 , Yu-Feng Song 1 , Zhi Luo 1, 5
Affiliation
|
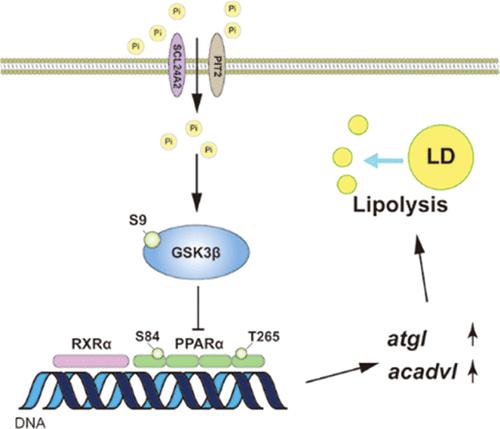
Excessive phosphorus (Pi) contributes to eutrophication in an aquatic environment, which threatens human and fish health. However, the mechanisms by which Pi overload influences aquatic animals remain largely unexplored. In the present study, Pi supplementation increased the Pi content, inhibited lipid accumulation and lipogenesis, and stimulated lipolysis in the liver. Pi supplementation increased the phosphorylation of glycogen synthase kinase-3 β (GSK3β) at serine 9 (S9) but inhibited the phosphorylation of GSK3α at tyrosine 279 (Y279), GSK3β at tyrosine 216 (Y216), and peroxisome proliferator-activated receptor α (PPARα) at serine 84 (S84) and threonine 265 (T265). Pi supplementation also upregulated PPARα protein expression and stimulated its transcriptional activity, thereby inducing lipolysis. Pi suppressed GSK3β activity and prevented GSK3β, but not GSK3α, from interacting with PPARα, which in turn alleviated PPARα phosphorylation. GSK3β-induced phosphorylation of PPARα was dependent on GSK3β S9 dephosphorylation rather than Y216 phosphorylation. Mechanistically, underphosphorylation of PPARα mediated Pi-induced lipid degradation through transcriptionally activating adipose triglyceride lipase (atgl) and very long-chain-specific acyl-CoA dehydrogenase (acadvl). Collectively, our findings uncovered a new mechanism by which Pi facilitates lipolysis via the GSK3β–PPARα pathway and highlighted the importance of S84 and T265 phosphorylation in PPARα action.
中文翻译:

磷超载通过抑制淡水硬骨鱼中 PPARα 在 Ser84 和 Thr265 位点的 GSK3β 依赖性磷酸化来促进肝脏脂肪分解
过量的磷 (Pi) 会导致水生环境富营养化,从而威胁人类和鱼类的健康。然而,Pi 超载影响水生动物的机制在很大程度上仍未得到探索。在本研究中,补充 Pi 可增加 Pi 含量,抑制脂质积累和脂肪生成,并刺激肝脏中的脂肪分解。补充 Pi 增加糖原合酶激酶 3 β (GSK3β) 在丝氨酸 9 (S9) 的磷酸化,但抑制 GSK3α 在酪氨酸 279 (Y279)、GSK3β 在酪氨酸 216 (Y216) 和过氧化物酶体增殖物激活受体 α 的磷酸化 ( PPARα) 在丝氨酸 84 (S84) 和苏氨酸 265 (T265)。补充 Pi 还上调 PPARα 蛋白表达并刺激其转录活性,从而诱导脂肪分解。Pi 抑制 GSK3β 活性并阻止 GSK3β 而不是 GSK3α 与 PPARα 相互作用,从而减轻 PPARα 磷酸化。GSK3β 诱导的 PPARα 磷酸化依赖于 GSK3β S9 去磷酸化而不是 Y216 磷酸化。从机制上讲,PPARα 的磷酸化不足通过转录激活脂肪甘油三酯脂肪酶介导 Pi 诱导的脂质降解(atgl ) 和非常长链特异性的酰基辅酶 A 脱氢酶 ( acadvl )。总的来说,我们的研究结果揭示了一种新机制,Pi通过GSK3β–PPARα 途径促进脂肪分解,并强调了 S84 和 T265 磷酸化在 PPARα 作用中的重要性。
更新日期:2023-02-02
中文翻译:

磷超载通过抑制淡水硬骨鱼中 PPARα 在 Ser84 和 Thr265 位点的 GSK3β 依赖性磷酸化来促进肝脏脂肪分解
过量的磷 (Pi) 会导致水生环境富营养化,从而威胁人类和鱼类的健康。然而,Pi 超载影响水生动物的机制在很大程度上仍未得到探索。在本研究中,补充 Pi 可增加 Pi 含量,抑制脂质积累和脂肪生成,并刺激肝脏中的脂肪分解。补充 Pi 增加糖原合酶激酶 3 β (GSK3β) 在丝氨酸 9 (S9) 的磷酸化,但抑制 GSK3α 在酪氨酸 279 (Y279)、GSK3β 在酪氨酸 216 (Y216) 和过氧化物酶体增殖物激活受体 α 的磷酸化 ( PPARα) 在丝氨酸 84 (S84) 和苏氨酸 265 (T265)。补充 Pi 还上调 PPARα 蛋白表达并刺激其转录活性,从而诱导脂肪分解。Pi 抑制 GSK3β 活性并阻止 GSK3β 而不是 GSK3α 与 PPARα 相互作用,从而减轻 PPARα 磷酸化。GSK3β 诱导的 PPARα 磷酸化依赖于 GSK3β S9 去磷酸化而不是 Y216 磷酸化。从机制上讲,PPARα 的磷酸化不足通过转录激活脂肪甘油三酯脂肪酶介导 Pi 诱导的脂质降解(atgl ) 和非常长链特异性的酰基辅酶 A 脱氢酶 ( acadvl )。总的来说,我们的研究结果揭示了一种新机制,Pi通过GSK3β–PPARα 途径促进脂肪分解,并强调了 S84 和 T265 磷酸化在 PPARα 作用中的重要性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号