当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Cross-Metathesis Reactions That Afford E- and Z-Trisubstituted Alkenyl Bromides: Scope, Applications, and Mechanistic Insights
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-01 , DOI: 10.1021/jacs.2c13289 Tobias Koengeter 1 , Can Qin 2 , Binh Khanh Mai 3 , Qinghe Liu 1 , Yucheng Mu 1 , Peng Liu 3 , Amir H Hoveyda 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-01 , DOI: 10.1021/jacs.2c13289 Tobias Koengeter 1 , Can Qin 2 , Binh Khanh Mai 3 , Qinghe Liu 1 , Yucheng Mu 1 , Peng Liu 3 , Amir H Hoveyda 1, 2
Affiliation
|
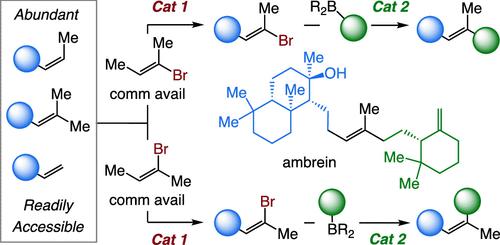
Stereochemically defined trisubstituted alkenes with a bromide and a methyl group at a terminus can be readily and stereoretentively derivatized through catalytic cross-coupling, affording unsaturated fragments found in many bioactive natural products. A direct method for generating such entities would be by stereocontrolled catalytic cross-metathesis (CM). Such methods are scarce however. Here, we present a stereoretentive strategy for CM between tri-, Z- or E-di, or monosubstituted olefins and Z- or E-2-bromo-2-butene, affording an assortment of E- or Z-trisubstituted alkenyl bromides. The majority of the transformations were catalyzed by two Mo monoaryloxide pyrrolide (MAP) complexes, one purchasable and the other accessible by well-established protocols. Substrates, such as feedstock trisubstituted olefins, can be purchased; the alkenyl bromide reagents are commercially available or can be prepared in two steps in a multigram scale. The catalytic process can be used to generate products that contain polar moieties, such as an amine or an alcohol, or sterically hindered alkenes that are α- or β-branched. The utility of the approach is highlighted by a brief and stereocontrolled synthesis of an unsaturated fragment of phomactin A and a concise total synthesis of ambrein. An unexpected outcome of these investigations was the discovery of a new role for the presence of a small-molecule alkene in an olefin metathesis reaction. DFT studies indicate that this additive swiftly reacts with a short-lived Mo alkylidene and probably helps circumvent the formation of catalytically inactive square pyramidal metallacyclobutanes, enhancing the efficiency of a transformation.
中文翻译:

提供 E-和 Z-三取代烯基溴的催化交叉复分解反应:范围、应用和机理见解
末端具有溴化物和甲基的立体化学定义的三取代烯烃可以通过催化交叉偶联轻松地立体保留衍生,从而提供在许多生物活性天然产物中发现的不饱和片段。生成此类实体的直接方法是通过立体控制催化交叉复分解(CM)。然而,这样的方法很少。在这里,我们提出了三-、 Z-或E-二或单取代烯烃与Z-或E -2-溴-2-丁烯之间的CM立体保留策略,提供了各种E-或Z-三取代烯基溴。大多数转化是由两种钼单芳氧基吡咯烷 (MAP) 络合物催化,一种可购买,另一种可通过完善的方案获得。底物,例如原料三取代烯烃,可以购买;烯基溴试剂是可商购的或者可以以多克规模分两步制备。催化过程可用于生成含有极性部分的产物,例如胺或醇,或α-或β-支化的空间位阻烯烃。该方法的实用性通过 phomactin A 不饱和片段的简短立体控制合成和龙涎香的简明全合成得到强调。这些研究的一个意想不到的结果是发现了小分子烯烃在烯烃复分解反应中的新作用。 DFT 研究表明,这种添加剂能与短寿命的 Mo 亚烷基快速反应,可能有助于避免催化惰性方锥金属环丁烷的形成,从而提高转化效率。
更新日期:2023-02-01
中文翻译:

提供 E-和 Z-三取代烯基溴的催化交叉复分解反应:范围、应用和机理见解
末端具有溴化物和甲基的立体化学定义的三取代烯烃可以通过催化交叉偶联轻松地立体保留衍生,从而提供在许多生物活性天然产物中发现的不饱和片段。生成此类实体的直接方法是通过立体控制催化交叉复分解(CM)。然而,这样的方法很少。在这里,我们提出了三-、 Z-或E-二或单取代烯烃与Z-或E -2-溴-2-丁烯之间的CM立体保留策略,提供了各种E-或Z-三取代烯基溴。大多数转化是由两种钼单芳氧基吡咯烷 (MAP) 络合物催化,一种可购买,另一种可通过完善的方案获得。底物,例如原料三取代烯烃,可以购买;烯基溴试剂是可商购的或者可以以多克规模分两步制备。催化过程可用于生成含有极性部分的产物,例如胺或醇,或α-或β-支化的空间位阻烯烃。该方法的实用性通过 phomactin A 不饱和片段的简短立体控制合成和龙涎香的简明全合成得到强调。这些研究的一个意想不到的结果是发现了小分子烯烃在烯烃复分解反应中的新作用。 DFT 研究表明,这种添加剂能与短寿命的 Mo 亚烷基快速反应,可能有助于避免催化惰性方锥金属环丁烷的形成,从而提高转化效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号