当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chelating Bis-silylenes As Powerful Ligands To Enable Unusual Low-Valent Main-Group Element Functions
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2023-01-31 , DOI: 10.1021/acs.accounts.2c00763 Shenglai Yao 1 , Artemis Saddington 1 , Yun Xiong 1 , Matthias Driess 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2023-01-31 , DOI: 10.1021/acs.accounts.2c00763 Shenglai Yao 1 , Artemis Saddington 1 , Yun Xiong 1 , Matthias Driess 1
Affiliation
|
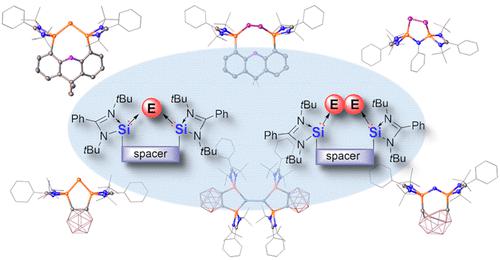
Silylenes are divalent silicon species with an unoccupied 3p orbital and one lone pair of electrons at the SiII center. Owing to the excellent σ-donating ability of amidinato-based silylenes, which stems from the intramolecular imino-N donor interaction with the vacant 3p orbital of the silicon atom, N-heterocyclic amidinato bis(silylenes) [bis(NHSi)s] can serve as versatile strong donating ligands for cooperative stabilization of central atoms in unusually low oxidation states. Herein, we present our recent achievement on the application of bis(NHSi) ligands with electronically and spatially different spacers to main-group chemistry, which has allowed the isolation of a variety of low-valent compounds consisting of monatomic zero-valent group 14 E0 complexes (named “metallylones”, E = Si, Ge, Sn, Pb); monovalent group 15 EI complexes (E = N, P, isoelectronic with metallylones); and diatomic low-valent E2 complexes (E = Si, Ge, P) with intriguing electronic structures and chemical reactivities.
中文翻译:

螯合双甲硅烷作为强大的配体以实现不寻常的低价主族元素功能
甲硅烷基是二价硅物质,具有未占据的 3p 轨道和 Si II中心的一对孤对电子。由于亚氨基基亚甲硅基具有优异的 σ 供体能力,这源于分子内亚氨基-N供体与硅原子的空 3p 轨道的相互作用,N-杂环亚氨基双(亚甲硅基)[bis(NHSi)s] 可以作为多功能的强配体,可在异常低的氧化态下协同稳定中心原子。在此,我们介绍了我们最近在将具有电子和空间不同间隔基的双 (NHSi) 配体应用于主族化学方面取得的成果,这使得可以分离由单原子零价组 14 E 组成的各种低价化合物0络合物(命名为“metallylones”,E = Si、Ge、Sn、Pb);单价组 15 E I配合物(E = N,P,与金属酮等电子);和具有有趣电子结构和化学反应性的双原子低价 E 2配合物(E = Si、Ge、P)。
更新日期:2023-01-31
中文翻译:

螯合双甲硅烷作为强大的配体以实现不寻常的低价主族元素功能
甲硅烷基是二价硅物质,具有未占据的 3p 轨道和 Si II中心的一对孤对电子。由于亚氨基基亚甲硅基具有优异的 σ 供体能力,这源于分子内亚氨基-N供体与硅原子的空 3p 轨道的相互作用,N-杂环亚氨基双(亚甲硅基)[bis(NHSi)s] 可以作为多功能的强配体,可在异常低的氧化态下协同稳定中心原子。在此,我们介绍了我们最近在将具有电子和空间不同间隔基的双 (NHSi) 配体应用于主族化学方面取得的成果,这使得可以分离由单原子零价组 14 E 组成的各种低价化合物0络合物(命名为“metallylones”,E = Si、Ge、Sn、Pb);单价组 15 E I配合物(E = N,P,与金属酮等电子);和具有有趣电子结构和化学反应性的双原子低价 E 2配合物(E = Si、Ge、P)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号