当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling the deposition/dissolution chemistry of MnO2 for high-energy aqueous batteries
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2023-02-01 , DOI: 10.1039/d3ee00018d Xiaolin Ye 1, 2, 3 , Daliang Han 1, 2, 3 , Guangyi Jiang 1, 2, 3 , Changjun Cui 1, 2, 3 , Yong Guo 1, 2, 3 , Yaogang Wang 1, 2, 3 , Zhicheng Zhang 1, 2, 3 , Zhe Weng 1, 2, 3 , Quan-Hong Yang 1, 2, 3
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2023-02-01 , DOI: 10.1039/d3ee00018d Xiaolin Ye 1, 2, 3 , Daliang Han 1, 2, 3 , Guangyi Jiang 1, 2, 3 , Changjun Cui 1, 2, 3 , Yong Guo 1, 2, 3 , Yaogang Wang 1, 2, 3 , Zhicheng Zhang 1, 2, 3 , Zhe Weng 1, 2, 3 , Quan-Hong Yang 1, 2, 3
Affiliation
|
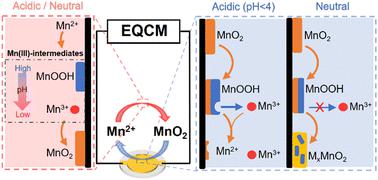
Aqueous rechargeable batteries based on the deposition/dissolution of MnO2 are drawing significant attention because of their record-high theoretical capacity and redox potential in addition to their low cost and high safety. However, the deposition/dissolution chemistry of MnO2 remains elusive, which must be overcome for it to be used in batteries. Herein, we used an in situ electrochemical quartz crystal microbalance technique to reveal the deposition/dissolution process, and unequivocally identified that it has pH-dependent Mn(III)-intermediates (MnOOH and Mn3+). In particular, the dissolved Mn3+ results in a loss of active species and thus greatly decreases the discharge capacity, Coulombic efficiency and cycling stability. As proof of this new understanding, introducing some redox mediators into the electrolyte effectively addresses this problem in a prototype Cu//MnO2 battery. Our work provides a new and significant insight into the deposition/dissolution chemistry of MnO2, which will promote the further exploration of high-energy aqueous batteries.
中文翻译:

揭示用于高能水系电池的 MnO2 的沉积/溶解化学
基于MnO 2沉积/溶解的水系可充电电池因其创纪录的高理论容量和氧化还原电位以及低成本和高安全性而备受关注。然而,MnO 2的沉积/溶解化学仍然难以捉摸,必须克服它才能用于电池。在此,我们使用原位电化学石英晶体微天平技术来揭示沉积/溶解过程,并明确确定它具有 pH 依赖性 Mn( III )-中间体(MnOOH 和 Mn 3+)。特别是溶解的 Mn 3+导致活性物质的损失,从而大大降低放电容量、库仑效率和循环稳定性。作为这一新认识的证明,在电解液中引入一些氧化还原介质有效地解决了原型 Cu//MnO 2电池中的这个问题。我们的工作为 MnO 2的沉积/溶解化学提供了新的重要见解,这将促进对高能水系电池的进一步探索。
更新日期:2023-02-01
中文翻译:

揭示用于高能水系电池的 MnO2 的沉积/溶解化学
基于MnO 2沉积/溶解的水系可充电电池因其创纪录的高理论容量和氧化还原电位以及低成本和高安全性而备受关注。然而,MnO 2的沉积/溶解化学仍然难以捉摸,必须克服它才能用于电池。在此,我们使用原位电化学石英晶体微天平技术来揭示沉积/溶解过程,并明确确定它具有 pH 依赖性 Mn( III )-中间体(MnOOH 和 Mn 3+)。特别是溶解的 Mn 3+导致活性物质的损失,从而大大降低放电容量、库仑效率和循环稳定性。作为这一新认识的证明,在电解液中引入一些氧化还原介质有效地解决了原型 Cu//MnO 2电池中的这个问题。我们的工作为 MnO 2的沉积/溶解化学提供了新的重要见解,这将促进对高能水系电池的进一步探索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号