当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Cleavage of the Dative Bond of Ammonia Borane by Polymeric Carbonyl Groups for Enhanced Energy Generation
Chemistry of Materials ( IF 7.2 ) Pub Date : 2023-01-31 , DOI: 10.1021/acs.chemmater.2c02684 Prithwish Biswas 1 , Yujie Wang 1 , Steven Herrera 1 , Pankaj Ghildiyal 1, 2 , Michael R. Zachariah 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2023-01-31 , DOI: 10.1021/acs.chemmater.2c02684 Prithwish Biswas 1 , Yujie Wang 1 , Steven Herrera 1 , Pankaj Ghildiyal 1, 2 , Michael R. Zachariah 1
Affiliation
|
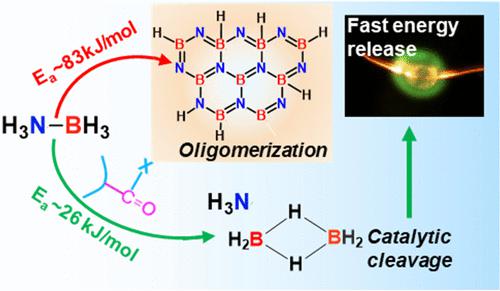
Dissociation of the dative (B–N) bond of ammonia borane (NH3BH3/AB) is essential to prevent its thermochemical oligomerization to chemically resistant BNHx compounds, for its applicability as a fuel in high-energy propulsion systems. We show that when AB is incorporated into polymer matrices containing carbonyl functional groups, thermal activation causes the carbonyl groups to engage in nucleophilic interactions with AB. Such interactions catalyze the lysis of the dative B–N bond resulting in the decomposition of AB to NH3 and B2H6 gases, with no evidence of oligomerization. We find that the carbonyl groups function as catalysts and do not participate in any net reaction. In situ high-heating rate (∼105 K/s) characterizations demonstrate that facile-synthesized hierarchical (micro/nano) composite particles of AB/carbonyl-based polymers completely gasify to NH3 and B2H6 at ∼510 K, followed by spontaneous ignition in the air with negligible delay. Thus, the current chemical pathway enables the solid-state storage of reactive fuels, NH3 and B2H6, and their controlled on-demand release for high-energy applications.
中文翻译:

聚合羰基催化裂解氨硼烷的配位键以增强能量产生
氨硼烷 (NH 3 BH 3 /AB)的配位 (B-N) 键的解离对于防止其热化学齐聚成耐化学 BNH x化合物至关重要,因为它可用作高能推进系统中的燃料。我们表明,当 AB 掺入含有羰基官能团的聚合物基质时,热活化会导致羰基与 AB 发生亲核相互作用。这种相互作用催化了配位 B-N 键的裂解,导致 AB 分解为 NH 3和 B 2 H 6气体,没有低聚的迹象。我们发现羰基起到催化剂的作用,不参与任何净反应。原位高加热速率(~10 5 K/s)表征表明,AB/羰基基聚合物的简易合成分级(微/纳米)复合颗粒在~510 K 时完全气化为 NH 3和 B 2 H 6,随后在空气中自燃,延迟可忽略不计。因此,当前的化学途径使反应燃料 NH 3和 B 2 H 6的固态存储成为可能,并且它们可控地按需释放以用于高能应用。
更新日期:2023-01-31
中文翻译:

聚合羰基催化裂解氨硼烷的配位键以增强能量产生
氨硼烷 (NH 3 BH 3 /AB)的配位 (B-N) 键的解离对于防止其热化学齐聚成耐化学 BNH x化合物至关重要,因为它可用作高能推进系统中的燃料。我们表明,当 AB 掺入含有羰基官能团的聚合物基质时,热活化会导致羰基与 AB 发生亲核相互作用。这种相互作用催化了配位 B-N 键的裂解,导致 AB 分解为 NH 3和 B 2 H 6气体,没有低聚的迹象。我们发现羰基起到催化剂的作用,不参与任何净反应。原位高加热速率(~10 5 K/s)表征表明,AB/羰基基聚合物的简易合成分级(微/纳米)复合颗粒在~510 K 时完全气化为 NH 3和 B 2 H 6,随后在空气中自燃,延迟可忽略不计。因此,当前的化学途径使反应燃料 NH 3和 B 2 H 6的固态存储成为可能,并且它们可控地按需释放以用于高能应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号