当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Ligand-Controlled Selective Reductive Cyclization/Cross-Couplings
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2023-01-23 , DOI: 10.1021/acs.accounts.2c00771 Qi Pan 1 , Yuanyuan Ping 1 , Wangqing Kong 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2023-01-23 , DOI: 10.1021/acs.accounts.2c00771 Qi Pan 1 , Yuanyuan Ping 1 , Wangqing Kong 1
Affiliation
|
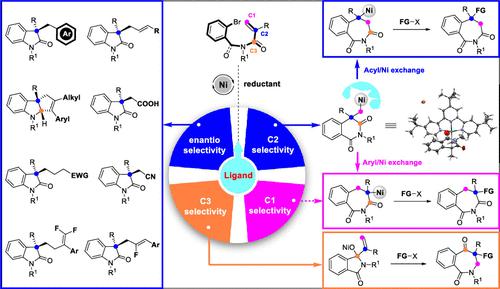
The use of quaternary stereocenters during lead candidate optimization continues to grow because of improved physiochemical and pharmacokinetic profiles of compounds with higher sp3 fraction. Pd-catalyzed redox-neutral alkene difunctionalization involving carbopalladation of alkenes followed by nucleophilic-trapping σ-alkyl-palladium intermediates has been developed as an efficient method to construct quaternary stereocenters. However, the low chemoselectivity and air sensitivity of organometallic nucleophiles, as well as their low availability and accessibility, limit the scope of application of this elegant strategy. Recently, Ni-catalyzed reductive cross-coupling has evolved into a privileged strategy to easily construct valuable C(sp3)–C bonds. Despite great progress, the enantioselective coupling of C(sp3) electrophiles still relies on activated or functionalized alkyl precursors, which are often unstable and require multiple steps to prepare. Therefore, Ni-catalyzed reductive difunctionalization of alkenes via selective cyclization/cross-coupling was developed. This strategy not only offers a robust and practical alternative for traditional redox-neutral alkene difunctionalization but also provides strategic complementarity for reductive cross-coupling of activated alkyl electrophiles. In this Account, we summarize the latest results from our laboratory on this topic. These findings mainly include our explorations in modulating the enantioselectivity and cyclization mode of reductive cyclization/cross-couplings.
中文翻译:

镍催化配体控制的选择性还原环化/交叉偶联
由于具有更高 sp 3分数的化合物的物理化学和药代动力学特征得到改善,在先导候选物优化过程中四元立构中心的使用持续增长。Pd 催化的氧化还原中性烯烃双官能化涉及烯烃的碳钯化,然后是亲核捕获的 σ-烷基-钯中间体,已被开发为构建季立体中心的有效方法。然而,有机金属亲核试剂的低化学选择性和空气敏感性,以及它们的低可用性和可及性,限制了这种优雅策略的应用范围。最近,Ni 催化的还原交叉偶联已发展成为一种特权策略,可以轻松构建有价值的 C(sp 3)–C键。尽管取得了很大进展,但 C(sp 3 )亲电子试剂的对映选择性偶联仍然依赖于活化或功能化的烷基前体,这些前体通常不稳定,需要多个步骤才能制备。因此,开发了通过选择性环化/交叉偶联的镍催化烯烃还原双官能化。该策略不仅为传统的氧化还原-中性烯烃双官能化提供了一种可靠且实用的替代方案,而且还为活化的烷基亲电子试剂的还原交叉偶联提供了战略互补性。在此帐户中,我们总结了我们实验室在该主题上的最新结果。这些发现主要包括我们在调节还原环化/交叉偶联的对映选择性和环化模式方面的探索。
更新日期:2023-01-23
中文翻译:

镍催化配体控制的选择性还原环化/交叉偶联
由于具有更高 sp 3分数的化合物的物理化学和药代动力学特征得到改善,在先导候选物优化过程中四元立构中心的使用持续增长。Pd 催化的氧化还原中性烯烃双官能化涉及烯烃的碳钯化,然后是亲核捕获的 σ-烷基-钯中间体,已被开发为构建季立体中心的有效方法。然而,有机金属亲核试剂的低化学选择性和空气敏感性,以及它们的低可用性和可及性,限制了这种优雅策略的应用范围。最近,Ni 催化的还原交叉偶联已发展成为一种特权策略,可以轻松构建有价值的 C(sp 3)–C键。尽管取得了很大进展,但 C(sp 3 )亲电子试剂的对映选择性偶联仍然依赖于活化或功能化的烷基前体,这些前体通常不稳定,需要多个步骤才能制备。因此,开发了通过选择性环化/交叉偶联的镍催化烯烃还原双官能化。该策略不仅为传统的氧化还原-中性烯烃双官能化提供了一种可靠且实用的替代方案,而且还为活化的烷基亲电子试剂的还原交叉偶联提供了战略互补性。在此帐户中,我们总结了我们实验室在该主题上的最新结果。这些发现主要包括我们在调节还原环化/交叉偶联的对映选择性和环化模式方面的探索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号