当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic and Kinetic Barriers Limiting Solid-State Reactions Resolved through In Situ Synchrotron Studies of Lithium Halide Salts
Chemistry of Materials ( IF 7.2 ) Pub Date : 2023-01-20 , DOI: 10.1021/acs.chemmater.2c02543 Monty R. Cosby 1 , Christopher J. Bartel 2, 3 , Adam A. Corrao 1 , Andrey A. Yakovenko 4 , Leighanne C. Gallington 4 , Gerbrand Ceder 2, 3 , Peter G. Khalifah 1, 5
Chemistry of Materials ( IF 7.2 ) Pub Date : 2023-01-20 , DOI: 10.1021/acs.chemmater.2c02543 Monty R. Cosby 1 , Christopher J. Bartel 2, 3 , Adam A. Corrao 1 , Andrey A. Yakovenko 4 , Leighanne C. Gallington 4 , Gerbrand Ceder 2, 3 , Peter G. Khalifah 1, 5
Affiliation
|
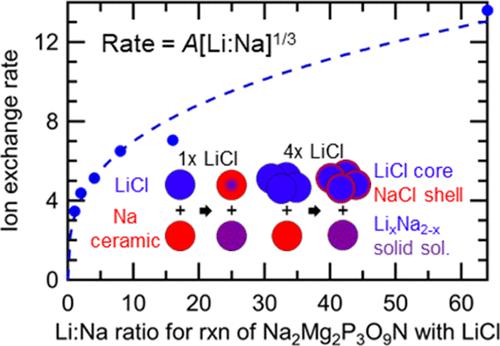
Although halide salts such as LiCl and LiBr are routinely used as a source of Li ions during ion exchange reactions, a detailed understanding of the processes controlling the rates of these reactions is presently lacking. Recently, we discovered that the rate-limiting barriers for ion exchange are commonly associated with these salts rather than the ceramic target of ion exchange, making it important to quantitatively understand salt processes. Here, it is demonstrated that in situ synchrotron studies of ion exchange reactions can be used to precisely quantify the thermodynamic activation energies associated with these solid-state reactions in a manner that can be directly compared with predictions from density functional theory (DFT). While the temperature dependence of the LiCl reaction rate is found to be set by a barrier associated with ion hopping, it was discovered that for LiBr, the rate is also affected by the defect formation energy─an energy found to be substantially lower than predicted by DFT. Furthermore, it is shown that by varying the relative amounts of reactants, the resulting change in reaction rate can be used to identify the rate-limiting reagent and to elucidate an overall scaling relationship that controls the concentration dependence of the reaction rate. Also, it is demonstrated that global fits across doped and undoped salts can be used to probe both intrinsic and extrinsic vacancy concentrations. This improved understanding of ion exchange mechanisms can be used to predict reaction conditions that can accelerate ion exchange reaction rates by orders of magnitude. The techniques demonstrated here can be broadly applied to probe the kinetics and thermodynamics of solid-state reactions.
中文翻译:

通过卤化锂盐的原位同步加速器研究解决了限制固态反应的热力学和动力学障碍
尽管氯化锂和溴化锂等卤化物盐在离子交换反应中通常用作锂离子源,但目前尚缺乏对控制这些反应速率的过程的详细了解。最近,我们发现离子交换的限速屏障通常与这些盐相关,而不是离子交换的陶瓷靶,因此定量了解盐过程非常重要。在这里,证明离子交换反应的原位同步加速器研究可用于精确量化与这些固态反应相关的热力学活化能,其方式可以直接与密度泛函理论 (DFT) 的预测进行比较。虽然发现 LiCl 反应速率的温度依赖性是由与离子跳跃相关的势垒设定的,但人们发现,对于 LiBr,反应速率也受缺陷形成能的影响——发现该能量大大低于预测的能量干膜傅立叶变换。此外,结果表明,通过改变反应物的相对量,由此产生的反应速率变化可用于识别限速试剂,并阐明控制反应速率浓度依赖性的整体比例关系。此外,还证明了掺杂和未掺杂盐的全局拟合可用于探测内在和外在空位浓度。这种对离子交换机制的改进理解可用于预测可将离子交换反应速率加快几个数量级的反应条件。这里展示的技术可以广泛应用于探测固态反应的动力学和热力学。
更新日期:2023-01-20
中文翻译:

通过卤化锂盐的原位同步加速器研究解决了限制固态反应的热力学和动力学障碍
尽管氯化锂和溴化锂等卤化物盐在离子交换反应中通常用作锂离子源,但目前尚缺乏对控制这些反应速率的过程的详细了解。最近,我们发现离子交换的限速屏障通常与这些盐相关,而不是离子交换的陶瓷靶,因此定量了解盐过程非常重要。在这里,证明离子交换反应的原位同步加速器研究可用于精确量化与这些固态反应相关的热力学活化能,其方式可以直接与密度泛函理论 (DFT) 的预测进行比较。虽然发现 LiCl 反应速率的温度依赖性是由与离子跳跃相关的势垒设定的,但人们发现,对于 LiBr,反应速率也受缺陷形成能的影响——发现该能量大大低于预测的能量干膜傅立叶变换。此外,结果表明,通过改变反应物的相对量,由此产生的反应速率变化可用于识别限速试剂,并阐明控制反应速率浓度依赖性的整体比例关系。此外,还证明了掺杂和未掺杂盐的全局拟合可用于探测内在和外在空位浓度。这种对离子交换机制的改进理解可用于预测可将离子交换反应速率加快几个数量级的反应条件。这里展示的技术可以广泛应用于探测固态反应的动力学和热力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号