当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Double click macrocyclization with Sondheimer diyne of aza-dipyrrins for B–Free bioorthogonal imaging
Chemical Communications ( IF 4.3 ) Pub Date : 2023-01-20 , DOI: 10.1039/d2cc06461h Dan Wu 1 , Gonzalo Durán-Sampedro 1 , Sheila Fitzgerald 1 , Massimiliano Garre 1 , Donal F O'Shea 1
Chemical Communications ( IF 4.3 ) Pub Date : 2023-01-20 , DOI: 10.1039/d2cc06461h Dan Wu 1 , Gonzalo Durán-Sampedro 1 , Sheila Fitzgerald 1 , Massimiliano Garre 1 , Donal F O'Shea 1
Affiliation
|
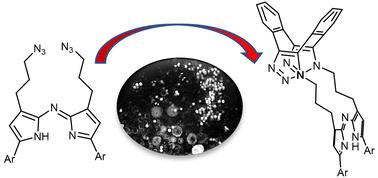
Sequential azide/diyne cycloadditions proved highly effective for the macrocyclization of a bis-azido aza-dipyrrin. Macrocyclic aza-dipyrrin could be produced in 30 min at rt in water with changes in fluorescence intensity and lifetimes measurable upon reaction. Live cell microscopy showed that aza-dipyrrins were suitable for confocal and STED super-resolution imaging and a bioorthogonal response to macrocyclization could be detected in cellular compartments. These results will encourage a broader examination of the sensing and imaging uses of aza-dipyrrins.
中文翻译:

用氮杂联吡啶的 Sondheimer 二炔双击大环化用于无 B 生物正交成像
顺序叠氮化物/二炔环加成被证明对双叠氮基氮杂联吡啶的大环化非常有效。大环氮杂联吡啶可在室温下于水中在 30 分钟内生成,并可在反应时测量荧光强度和寿命的变化。活细胞显微镜显示氮杂联吡啶适用于共聚焦和 STED 超分辨率成像,并且可以在细胞区室中检测到对大环化的生物正交反应。这些结果将鼓励对氮杂联吡啶的传感和成像用途进行更广泛的研究。
更新日期:2023-01-20
中文翻译:

用氮杂联吡啶的 Sondheimer 二炔双击大环化用于无 B 生物正交成像
顺序叠氮化物/二炔环加成被证明对双叠氮基氮杂联吡啶的大环化非常有效。大环氮杂联吡啶可在室温下于水中在 30 分钟内生成,并可在反应时测量荧光强度和寿命的变化。活细胞显微镜显示氮杂联吡啶适用于共聚焦和 STED 超分辨率成像,并且可以在细胞区室中检测到对大环化的生物正交反应。这些结果将鼓励对氮杂联吡啶的传感和成像用途进行更广泛的研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号