当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of a Pyridoazepine Scaffold via Rhodium-Catalyzed Ring Expansion and Nitroacetamide Condensation
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-01-16 , DOI: 10.1021/acs.oprd.2c00145 Eddie Yang 1 , Joseph W. Tucker 1 , Thomas A. Chappie 2 , John D. Weaver 1 , Caroline Chapman 3 , Remzi Duzguner 1 , John M. Humphrey 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-01-16 , DOI: 10.1021/acs.oprd.2c00145 Eddie Yang 1 , Joseph W. Tucker 1 , Thomas A. Chappie 2 , John D. Weaver 1 , Caroline Chapman 3 , Remzi Duzguner 1 , John M. Humphrey 1
Affiliation
|
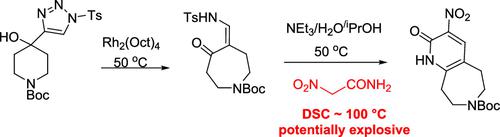
Pyridoazepines are privileged structures in the search for novel drug candidates. We present the synthesis of a versatile pyridoazepine scaffold employing a precedented rhodium(II)-catalyzed ring expansion and subsequent double-condensation-cyclization with nitroacetamide. The reactions applied several potentially hazardous reagents and intermediates, including p-toluenesulfonyl azide and nitroacetamide, the latter of which was determined to be potentially explosive and shock-sensitive. Reactions employed were derisked for laboratory scale work through DSC and TSU analysis in concert with optimization of reaction conditions. The key rhodium-catalyzed ring expansion was shown by TSU analysis to occur in a controllable manner with manageable nitrogen evolution. A new procedure is presented that enables preparation of nitroacetamide without excessive manipulation (i.e., extractive isolation) or isolation of dry solid. This article describes early safety studies used to derisk the preparation of multi-gram quantities of the target pyridoazepine.
中文翻译:

通过铑催化的扩环和硝基乙酰胺缩合合成吡啶并氮卓支架
吡啶并氮卓类化合物是寻找新型候选药物的特殊结构。我们介绍了一种多功能吡啶并氮卓支架的合成,该支架采用先行的铑 (II) 催化的扩环和随后与硝基乙酰胺的双缩合环化。该反应使用了几种具有潜在危险的试剂和中间体,包括对甲苯磺酰叠氮化物和硝基乙酰胺,后者被确定为具有潜在的爆炸性和冲击敏感性。通过 DSC 和 TS U分析结合反应条件的优化,对所采用的反应进行实验室规模工作的风险降低。TS U显示了关键的铑催化环扩展分析以可控的方式进行,氮的释放可控。提出了一种新程序,无需过度操作(即萃取分离)或干燥固体分离即可制备硝基乙酰胺。本文介绍了早期的安全性研究,这些研究用于降低多克量目标吡啶并氮平制剂的风险。
更新日期:2023-01-16
中文翻译:

通过铑催化的扩环和硝基乙酰胺缩合合成吡啶并氮卓支架
吡啶并氮卓类化合物是寻找新型候选药物的特殊结构。我们介绍了一种多功能吡啶并氮卓支架的合成,该支架采用先行的铑 (II) 催化的扩环和随后与硝基乙酰胺的双缩合环化。该反应使用了几种具有潜在危险的试剂和中间体,包括对甲苯磺酰叠氮化物和硝基乙酰胺,后者被确定为具有潜在的爆炸性和冲击敏感性。通过 DSC 和 TS U分析结合反应条件的优化,对所采用的反应进行实验室规模工作的风险降低。TS U显示了关键的铑催化环扩展分析以可控的方式进行,氮的释放可控。提出了一种新程序,无需过度操作(即萃取分离)或干燥固体分离即可制备硝基乙酰胺。本文介绍了早期的安全性研究,这些研究用于降低多克量目标吡啶并氮平制剂的风险。











































 京公网安备 11010802027423号
京公网安备 11010802027423号