当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
O-GalNAc glycosylation affects the immunogenicity of the receptor-binding domain (RBD) of SARS-CoV-2 spike protein
Chemical Communications ( IF 4.3 ) Pub Date : 2023-01-16 , DOI: 10.1039/d2cc06583e Yongheng Rong 1 , Xingyun Wang 2 , Weian Mao 1 , Min Chen 1 , Shengjun Wang 3 , Peng George Wang 2 , Yunjiao He 2 , Yun Kong 1
Chemical Communications ( IF 4.3 ) Pub Date : 2023-01-16 , DOI: 10.1039/d2cc06583e Yongheng Rong 1 , Xingyun Wang 2 , Weian Mao 1 , Min Chen 1 , Shengjun Wang 3 , Peng George Wang 2 , Yunjiao He 2 , Yun Kong 1
Affiliation
|
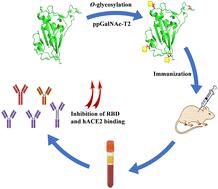
The spike protein of SARS-CoV-2 has been widely used as an effective vaccine immunogen, although some limitations still remain. Herein, O-GalNAc glycosylated RBD (Tn-RBD) was synthesized as an antigen via in vitro glycosylation reactions. The inhibition ability against hACE2 binding of antibodies induced with Tn-RBD was 30–40% increased compared to that induced with RBD. This result implies that Tn-glycosylation might play important roles in the immunogenicity of the RBD protein, which should be considered in the design of novel vaccines to fight against COVID-19.
中文翻译:

O-GalNAc 糖基化影响 SARS-CoV-2 刺突蛋白受体结合域 (RBD) 的免疫原性
SARS-CoV-2 的刺突蛋白已被广泛用作有效的疫苗免疫原,但仍存在一些局限性。在此,O -GalNAc 糖基化 RBD (Tn-RBD)通过体外糖基化反应合成为抗原。与用 RBD 诱导的抗体相比,用 Tn-RBD 诱导的抗体对 hACE2 结合的抑制能力增加了 30-40%。这一结果表明 Tn-糖基化可能在 RBD 蛋白的免疫原性中发挥重要作用,在设计抗 COVID-19 的新型疫苗时应考虑这一点。
更新日期:2023-01-16
中文翻译:

O-GalNAc 糖基化影响 SARS-CoV-2 刺突蛋白受体结合域 (RBD) 的免疫原性
SARS-CoV-2 的刺突蛋白已被广泛用作有效的疫苗免疫原,但仍存在一些局限性。在此,O -GalNAc 糖基化 RBD (Tn-RBD)通过体外糖基化反应合成为抗原。与用 RBD 诱导的抗体相比,用 Tn-RBD 诱导的抗体对 hACE2 结合的抑制能力增加了 30-40%。这一结果表明 Tn-糖基化可能在 RBD 蛋白的免疫原性中发挥重要作用,在设计抗 COVID-19 的新型疫苗时应考虑这一点。









































 京公网安备 11010802027423号
京公网安备 11010802027423号