当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Formal Carbene Insertion into Carbon-Boron Bonds of Diboronates by N-Trisylhydrazones
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-12-28 , DOI: 10.1002/anie.202216356 Zhicheng Bao 1 , Meirong Huang 2, 3 , Yan Xu 1 , Xinhao Zhang 2, 3 , Yun-Dong Wu 2, 3 , Jianbo Wang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-12-28 , DOI: 10.1002/anie.202216356 Zhicheng Bao 1 , Meirong Huang 2, 3 , Yan Xu 1 , Xinhao Zhang 2, 3 , Yun-Dong Wu 2, 3 , Jianbo Wang 1
Affiliation
|
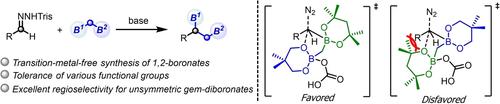
Intermolecular reaction of N-trisylhydrazones with bis (boryl) methane led to the transformation of 1,1-diboronate into 1,2-bis(boronates) via 1,2-borylmethyl migration. With unsymmetric diboronates with two different boryl moieties, the reaction proceeded with excellent regioselectivity. DFT studies reveal an unusual neighbouring boryl group effect that accounts for the observed regioselectivity.
中文翻译:

N-Trisylhydrazones 将卡宾选择性地插入二硼酸酯的碳-硼键中
N-三苯甲基腙与双(硼基)甲烷的分子间反应导致 1,1-二硼酸酯通过 1,2-硼基甲基迁移转化为 1,2-双(硼酸酯)。对于具有两个不同硼基部分的不对称二硼酸酯,反应以出色的区域选择性进行。DFT 研究揭示了一种不寻常的相邻硼基效应,该效应解释了观察到的区域选择性。
更新日期:2022-12-28
中文翻译:

N-Trisylhydrazones 将卡宾选择性地插入二硼酸酯的碳-硼键中
N-三苯甲基腙与双(硼基)甲烷的分子间反应导致 1,1-二硼酸酯通过 1,2-硼基甲基迁移转化为 1,2-双(硼酸酯)。对于具有两个不同硼基部分的不对称二硼酸酯,反应以出色的区域选择性进行。DFT 研究揭示了一种不寻常的相邻硼基效应,该效应解释了观察到的区域选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号