当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multifunctional Photo-Cross-Linking Probes: From Target Protein Searching to Imaging Applications
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-12-19 , DOI: 10.1021/acs.accounts.2c00505 Kostiantyn Kozoriz 1 , Olha Shkel 2 , Kyung Tae Hong 2 , Dong Hoon Kim 1 , Yun Kyung Kim 2 , Jun-Seok Lee 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-12-19 , DOI: 10.1021/acs.accounts.2c00505 Kostiantyn Kozoriz 1 , Olha Shkel 2 , Kyung Tae Hong 2 , Dong Hoon Kim 1 , Yun Kyung Kim 2 , Jun-Seok Lee 1
Affiliation
|
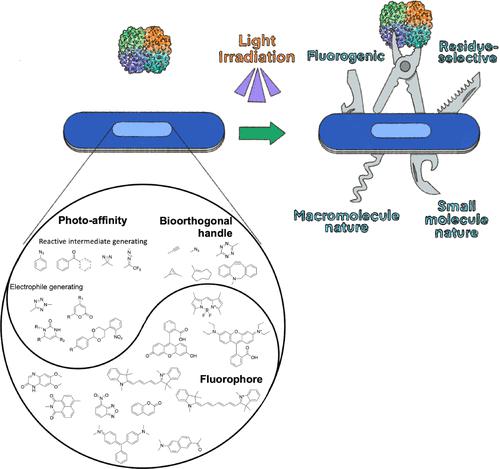
Despite advances in genome sequencing technology, the complete molecular interaction networks reflecting the biological functions of gene products have not been fully elucidated due to the lack of robust molecular interactome profiling techniques. Traditionally, molecular interactions have been investigated in vitro by measuring their affinity. However, such a reductionist approach comes with throughput constraints and does not depict an intact living cell environment. Therefore, molecular interactions in live cells must be captured to minimize false-positive results. The photo-cross-linking technique is a promising tool because the production of a temporally controlled reactive functional group can be induced using light exposure. Photoaffinity labeling is used in biochemistry and medicinal chemistry for bioconjugation, including drug and antibody conjugation, target protein identification of bioactive compounds, and fluorescent labeling of target proteins. This Account summarizes recent advances in multifunctional photo-cross-linkers for drug target identification and bioimaging. In addition to our group’s contributions, we reviewed the most notable examples from the last few decades to provide a comprehensive overview of how this field is evolving. Based on cross-linking chemistry, photo-cross-linkers are classified as either (i) reactive intermediate-generating or (ii) electrophile-generating. Reactive intermediates generating photoaffinity tags have been extensively modified to target a molecule of interest using aryl azide, benzophenone, diazirine, diazo, and acyl silanes. These species are highly reactive and can form covalent bonds, irrespective of residue. Their short lifetime is ideal for the instant capture and labeling of biomolecules. Recently, photocaged electrophiles have been investigated to take advantage of their residue selectivity and relatively high yield for adduct formation with tetrazole, nitrobenzyl alcohol, o-nitrophenylethylene, pyrone, and pyrimidone. Multifunctional photo-cross-linkers for two parallel practical applications have been developed using both classes of photoactivatable groups. Unbiased target interactome profiling of small-molecule drugs requires a challenging structure–activity relationship study (SAR) step to retain the nature or biological activity of the lead compound, which led to the design of a multifunctional “minimalist tag” comprising a bio-orthogonal handle, a photoaffinity labeling group, and functional groups to load target molecules. In contrast, fluorogenic photo-cross-linking is advantageous for bioimaging because it does not require an additional bio-orthogonal reaction to introduce a fluorophore to the minimalist tag. Our group has made progress on minimalist tags and fluorogenic photo-cross-linkers through fruitful collaborations with other groups. The current range of photoactivation reactions and applications demonstrate that photoaffinity tags can be improved. We expect exciting days in the rational design of new multifunctional photo-cross-linkers, particularly clinically interesting versions used in photodynamic or photothermal therapy.
中文翻译:

多功能光交联探针:从目标蛋白搜索到成像应用
尽管基因组测序技术取得了进步,但由于缺乏可靠的分子相互作用组分析技术,尚未完全阐明反映基因产物生物学功能的完整分子相互作用网络。传统上,分子相互作用是在体外研究的通过测量它们的亲和力。然而,这种还原论方法具有吞吐量限制,并且没有描绘出完整的活细胞环境。因此,必须捕获活细胞中的分子相互作用,以尽量减少假阳性结果。光交联技术是一种很有前途的工具,因为可以使用曝光来诱导时间控制的反应性官能团的产生。光亲和标记在生物化学和药物化学中用于生物偶联,包括药物和抗体偶联、生物活性化合物的靶蛋白鉴定以及靶蛋白的荧光标记。该帐户总结了用于药物靶标识别和生物成像的多功能光交联剂的最新进展。除了我们小组的贡献,我们回顾了过去几十年最著名的例子,以全面概述该领域的发展情况。基于交联化学,光交联剂分为 (i) 反应性中间体生成或 (ii) 亲电试剂生成。使用芳基叠氮化物、二苯甲酮、二氮杂环丙烷、重氮和酰基硅烷,对产生光亲和标签的反应性中间体进行了广泛的修饰,以靶向目标分子。这些物种具有很高的反应性,可以形成共价键,与残基无关。它们的短寿命非常适合即时捕获和标记生物分子。最近,已经研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,基于交联化学,光交联剂分为 (i) 反应性中间体生成或 (ii) 亲电试剂生成。使用芳基叠氮化物、二苯甲酮、二氮杂环丙烷、重氮和酰基硅烷,对产生光亲和标签的反应性中间体进行了广泛的修饰,以靶向目标分子。这些物种具有很高的反应性,可以形成共价键,与残基无关。它们的短寿命非常适合即时捕获和标记生物分子。最近,已经研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,基于交联化学,光交联剂分为 (i) 反应性中间体生成或 (ii) 亲电试剂生成。使用芳基叠氮化物、二苯甲酮、二氮杂环丙烷、重氮和酰基硅烷,对产生光亲和标签的反应性中间体进行了广泛的修饰,以靶向目标分子。这些物种具有很高的反应性,可以形成共价键,与残基无关。它们的短寿命非常适合即时捕获和标记生物分子。最近,已经研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,使用芳基叠氮化物、二苯甲酮、二氮杂环丙烷、重氮和酰基硅烷,对生成光亲和标签的反应性中间体进行了广泛修改,以靶向感兴趣的分子。这些物种具有很高的反应性,可以形成共价键,与残基无关。它们的短寿命非常适合即时捕获和标记生物分子。最近,研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,使用芳基叠氮化物、二苯甲酮、二氮杂环丙烷、重氮和酰基硅烷,对产生光亲和标签的反应性中间体进行了广泛的修饰,以靶向目标分子。这些物种具有很高的反应性,可以形成共价键,与残基无关。它们的短寿命非常适合即时捕获和标记生物分子。最近,已经研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,它们的短寿命非常适合即时捕获和标记生物分子。最近,已经研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,它们的短寿命非常适合即时捕获和标记生物分子。最近,研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,o-硝基苯乙烯、吡喃酮和嘧啶酮。已经使用两类光活化基团开发了用于两个平行实际应用的多功能光交联剂。小分子药物的无偏目标相互作用组分析需要具有挑战性的结构-活性关系研究 (SAR) 步骤来保留先导化合物的性质或生物活性,这导致设计了一个包含生物正交的多功能“极简标签”句柄、光亲和标记基团和用于加载目标分子的官能团。相比之下,荧光光交联有利于生物成像,因为它不需要额外的生物正交反应来将荧光团引入极简标签。通过与其他小组富有成效的合作,我们小组在极简标签和荧光光交联剂方面取得了进展。当前范围的光活化反应和应用表明可以改进光亲和标签。我们期待在合理设计新的多功能光交联剂方面有激动人心的日子,特别是用于光动力或光热疗法的具有临床意义的版本。
更新日期:2022-12-19
中文翻译:

多功能光交联探针:从目标蛋白搜索到成像应用
尽管基因组测序技术取得了进步,但由于缺乏可靠的分子相互作用组分析技术,尚未完全阐明反映基因产物生物学功能的完整分子相互作用网络。传统上,分子相互作用是在体外研究的通过测量它们的亲和力。然而,这种还原论方法具有吞吐量限制,并且没有描绘出完整的活细胞环境。因此,必须捕获活细胞中的分子相互作用,以尽量减少假阳性结果。光交联技术是一种很有前途的工具,因为可以使用曝光来诱导时间控制的反应性官能团的产生。光亲和标记在生物化学和药物化学中用于生物偶联,包括药物和抗体偶联、生物活性化合物的靶蛋白鉴定以及靶蛋白的荧光标记。该帐户总结了用于药物靶标识别和生物成像的多功能光交联剂的最新进展。除了我们小组的贡献,我们回顾了过去几十年最著名的例子,以全面概述该领域的发展情况。基于交联化学,光交联剂分为 (i) 反应性中间体生成或 (ii) 亲电试剂生成。使用芳基叠氮化物、二苯甲酮、二氮杂环丙烷、重氮和酰基硅烷,对产生光亲和标签的反应性中间体进行了广泛的修饰,以靶向目标分子。这些物种具有很高的反应性,可以形成共价键,与残基无关。它们的短寿命非常适合即时捕获和标记生物分子。最近,已经研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,基于交联化学,光交联剂分为 (i) 反应性中间体生成或 (ii) 亲电试剂生成。使用芳基叠氮化物、二苯甲酮、二氮杂环丙烷、重氮和酰基硅烷,对产生光亲和标签的反应性中间体进行了广泛的修饰,以靶向目标分子。这些物种具有很高的反应性,可以形成共价键,与残基无关。它们的短寿命非常适合即时捕获和标记生物分子。最近,已经研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,基于交联化学,光交联剂分为 (i) 反应性中间体生成或 (ii) 亲电试剂生成。使用芳基叠氮化物、二苯甲酮、二氮杂环丙烷、重氮和酰基硅烷,对产生光亲和标签的反应性中间体进行了广泛的修饰,以靶向目标分子。这些物种具有很高的反应性,可以形成共价键,与残基无关。它们的短寿命非常适合即时捕获和标记生物分子。最近,已经研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,使用芳基叠氮化物、二苯甲酮、二氮杂环丙烷、重氮和酰基硅烷,对生成光亲和标签的反应性中间体进行了广泛修改,以靶向感兴趣的分子。这些物种具有很高的反应性,可以形成共价键,与残基无关。它们的短寿命非常适合即时捕获和标记生物分子。最近,研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,使用芳基叠氮化物、二苯甲酮、二氮杂环丙烷、重氮和酰基硅烷,对产生光亲和标签的反应性中间体进行了广泛的修饰,以靶向目标分子。这些物种具有很高的反应性,可以形成共价键,与残基无关。它们的短寿命非常适合即时捕获和标记生物分子。最近,已经研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,它们的短寿命非常适合即时捕获和标记生物分子。最近,已经研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,它们的短寿命非常适合即时捕获和标记生物分子。最近,研究了光笼亲电子试剂,以利用它们的残基选择性和相对较高的产率与四唑、硝基苄醇形成加合物,o-硝基苯乙烯、吡喃酮和嘧啶酮。已经使用两类光活化基团开发了用于两个平行实际应用的多功能光交联剂。小分子药物的无偏目标相互作用组分析需要具有挑战性的结构-活性关系研究 (SAR) 步骤来保留先导化合物的性质或生物活性,这导致设计了一个包含生物正交的多功能“极简标签”句柄、光亲和标记基团和用于加载目标分子的官能团。相比之下,荧光光交联有利于生物成像,因为它不需要额外的生物正交反应来将荧光团引入极简标签。通过与其他小组富有成效的合作,我们小组在极简标签和荧光光交联剂方面取得了进展。当前范围的光活化反应和应用表明可以改进光亲和标签。我们期待在合理设计新的多功能光交联剂方面有激动人心的日子,特别是用于光动力或光热疗法的具有临床意义的版本。











































 京公网安备 11010802027423号
京公网安备 11010802027423号