当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile Assembly of Modular-Type Phosphines for Tackling Modern Arylation Processes
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-12-06 , DOI: 10.1021/acs.accounts.2c00587 Man Ho Tse 1, 2 , Pui Ying Choy 1, 2 , Fuk Yee Kwong 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-12-06 , DOI: 10.1021/acs.accounts.2c00587 Man Ho Tse 1, 2 , Pui Ying Choy 1, 2 , Fuk Yee Kwong 1, 2
Affiliation
|
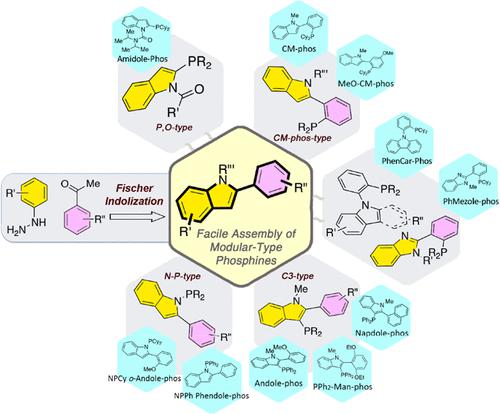
This Account presents an overview of a promising collection of phosphine ligands simply made from the modular Fischer indolization process and their applications in modern arylation processes. Using one easily accessible 2-arylindole scaffold, three major phosphino-moiety-positioned ligand series can be readily generated. We have attempted to explore challenging electrophilic and nucleophilic partners for the coupling reaction using the modular ligand tool. For the electrophilic partner study, CM-phos-type ligands, where the phosphino group is located at the 2-arene position of 2-arylindole, allow the successful cross-coupling of aryl mesylates. The CM-phos ligand forms a palladacycle before entering the cross-coupling catalytic cycle. For the nucleophilic partner investigation, the indole C3-positioned phosphines show the first accomplishment of Pd-catalyzed organotitanium nucleophile arylation. Indeed, the aryl-titanium nucleophile undergoes cross-coupling more efficiently than does the organoboron coupling partner in particular cases. Moreover, in the indole C3-positioned phosphine series, the −PPh2-containing ligands perform better in the highly sterically hindered cross-coupling of aryl chlorides than do ligands containing the −PCy2 moiety. The catalyst loading can even be reduced to 0.2 mol % Pd for tetra-ortho-substituted biaryl synthesis. This finding offers a new perspective on the next-generation design of phosphine ligands in which the sterically bulky and electron-rich −PR2 group (R = alkyl) may not be necessary for the cross-coupling of aryl chlorides. In general, we hypothesize that a good balance of steric and electronic properties for entertaining the oxidative addition and reductive elimination steps is crucial to the success of the reaction. For the steric factor, the highly sterically congested −PR2 group normally favors the reductive elimination, yet we conjecture that this sterically bulky group would serve as an obstacle for the incoming aryl halides. For the electronic factor, the electron rich −PR2 group is believed to support the oxidative cleavage of the C(Ar)–Cl bond by donating more electron density to the corresponding σ* orbital. Nevertheless, the high electron richness of the −PR2 group may disfavor the reductive elimination electronically. Overall, an appropriate balance of both electron density and steric bulkiness is suggested to allow the sterically hindered cross-coupling to proceed smoothly. We have found that the −PPh2-containing ligand is a good starting point for this investigation. The formation of aromatic carbon–carbon (C–C) and carbon–heteroatom (C–X) bonds from aryl chlorides was successfully realized using our proprietary phosphines.
中文翻译:

用于应对现代芳基化过程的模块化膦的简便组装
该帐户概述了一组有前途的膦配体,这些配体仅由模块化费歇尔吲哚化过程制成,及其在现代芳基化过程中的应用。使用一个容易获得的 2-芳基吲哚支架,可以很容易地生成三个主要的膦基部分定位配体系列。我们尝试使用模块化配体工具探索具有挑战性的亲电和亲核伙伴以进行偶联反应。对于亲电子伙伴研究,CM-phos型配体(其中膦基位于 2-芳基吲哚的 2-芳烃位置)允许甲磺酸芳基酯成功交叉偶联。CM-磷配体在进入交叉偶联催化循环之前形成钯环。对于亲核伙伴研究,吲哚 C3 位膦展示了 Pd 催化的有机钛亲核试剂芳基化的第一个成就。实际上,在特定情况下,芳基-钛亲核试剂比有机硼偶联伙伴更有效地进行交叉偶联。此外,在吲哚 C3 位膦系列中,与含有 -PCy 2部分的配体相比,含 -PPh 2的配体在芳基氯的高度空间位阻交叉偶联中表现更好。四邻位催化剂的催化剂负载量甚至可以减少到 0.2 mol% Pd-取代的联芳基合成。这一发现为下一代膦配体的设计提供了新的视角,其中空间体积庞大且富含电子的 −PR 2基团(R = 烷基)对于芳基氯的交叉偶联可能不是必需的。一般而言,我们假设用于进行氧化加成和还原消除步骤的空间和电子特性的良好平衡对于反应的成功至关重要。对于空间因子,高度空间拥挤的-PR 2基团通常有利于还原消除,但我们推测这个空间庞大的基团将成为进入芳基卤化物的障碍。对于电子因素,富电子-PR 2基团被认为通过向相应的 σ* 轨道提供更多的电子密度来支持 C(Ar)–Cl 键的氧化裂解。然而,-PR 2基团的高电子富集度可能不利于电子还原消除。总的来说,建议在电子密度和空间体积之间取得适当的平衡,以使空间位阻交叉偶联顺利进行。我们发现含-PPh 2的配体是这项研究的良好起点。使用我们专有的膦成功地实现了由芳基氯形成芳族碳-碳 (C-C) 和碳-杂原子 (C-X) 键。
更新日期:2022-12-06
中文翻译:

用于应对现代芳基化过程的模块化膦的简便组装
该帐户概述了一组有前途的膦配体,这些配体仅由模块化费歇尔吲哚化过程制成,及其在现代芳基化过程中的应用。使用一个容易获得的 2-芳基吲哚支架,可以很容易地生成三个主要的膦基部分定位配体系列。我们尝试使用模块化配体工具探索具有挑战性的亲电和亲核伙伴以进行偶联反应。对于亲电子伙伴研究,CM-phos型配体(其中膦基位于 2-芳基吲哚的 2-芳烃位置)允许甲磺酸芳基酯成功交叉偶联。CM-磷配体在进入交叉偶联催化循环之前形成钯环。对于亲核伙伴研究,吲哚 C3 位膦展示了 Pd 催化的有机钛亲核试剂芳基化的第一个成就。实际上,在特定情况下,芳基-钛亲核试剂比有机硼偶联伙伴更有效地进行交叉偶联。此外,在吲哚 C3 位膦系列中,与含有 -PCy 2部分的配体相比,含 -PPh 2的配体在芳基氯的高度空间位阻交叉偶联中表现更好。四邻位催化剂的催化剂负载量甚至可以减少到 0.2 mol% Pd-取代的联芳基合成。这一发现为下一代膦配体的设计提供了新的视角,其中空间体积庞大且富含电子的 −PR 2基团(R = 烷基)对于芳基氯的交叉偶联可能不是必需的。一般而言,我们假设用于进行氧化加成和还原消除步骤的空间和电子特性的良好平衡对于反应的成功至关重要。对于空间因子,高度空间拥挤的-PR 2基团通常有利于还原消除,但我们推测这个空间庞大的基团将成为进入芳基卤化物的障碍。对于电子因素,富电子-PR 2基团被认为通过向相应的 σ* 轨道提供更多的电子密度来支持 C(Ar)–Cl 键的氧化裂解。然而,-PR 2基团的高电子富集度可能不利于电子还原消除。总的来说,建议在电子密度和空间体积之间取得适当的平衡,以使空间位阻交叉偶联顺利进行。我们发现含-PPh 2的配体是这项研究的良好起点。使用我们专有的膦成功地实现了由芳基氯形成芳族碳-碳 (C-C) 和碳-杂原子 (C-X) 键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号