当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical synthesis of per-selenocysteine human epidermal growth factor
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2022-12-02 , DOI: 10.1002/psc.3464 Toshiki Takei 1 , Hideaki Tanaka 1 , Nobuaki Okumura 1 , Toshifumi Takao 1 , Luis Moroder 2 , Hironobu Hojo 1
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2022-12-02 , DOI: 10.1002/psc.3464 Toshiki Takei 1 , Hideaki Tanaka 1 , Nobuaki Okumura 1 , Toshifumi Takao 1 , Luis Moroder 2 , Hironobu Hojo 1
Affiliation
|
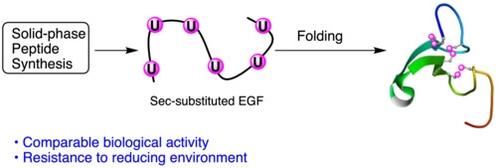
Human seleno-epidermal growth factor (seleno-EGF), a 53-residue peptide where all six cysteine residues of the parent human EGF sequence were replaced by selenocysteines, was synthesized and the oxidative folding of a polypeptide containing three diselenide bonds was compared to that of the parent cysteine peptide. The crude high performance liquid chromatography (HPLC) profiles clearly showed that both the native EGF and its selenocysteine-analogue fold smoothly, yielding a single sharp peak, proving that even in the case of three disulfide-bonded polypeptides the disulfide-to-diselenide bond substitution is highly isomorphous, as confirmed by conformational circular dichroism measurements and particularly by the biological assays.
中文翻译:

过硒代半胱氨酸人表皮生长因子的化学合成
人硒表皮生长因子 (seleno-EGF) 是一种 53 残基的肽,其中亲本人 EGF 序列的所有六个半胱氨酸残基都被硒代半胱氨酸取代,并且将含有三个二硒键的多肽的氧化折叠与母体半胱氨酸肽。粗制高效液相色谱 (HPLC) 图清楚地表明,天然 EGF 及其硒代半胱氨酸类似物折叠平稳,产生单个尖峰,证明即使在三个二硫键多肽的情况下,二硫键与二硒键取代是高度同构的,正如构象圆二色性测量,特别是生物学测定所证实的那样。
更新日期:2022-12-02
中文翻译:

过硒代半胱氨酸人表皮生长因子的化学合成
人硒表皮生长因子 (seleno-EGF) 是一种 53 残基的肽,其中亲本人 EGF 序列的所有六个半胱氨酸残基都被硒代半胱氨酸取代,并且将含有三个二硒键的多肽的氧化折叠与母体半胱氨酸肽。粗制高效液相色谱 (HPLC) 图清楚地表明,天然 EGF 及其硒代半胱氨酸类似物折叠平稳,产生单个尖峰,证明即使在三个二硫键多肽的情况下,二硫键与二硒键取代是高度同构的,正如构象圆二色性测量,特别是生物学测定所证实的那样。











































 京公网安备 11010802027423号
京公网安备 11010802027423号