当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
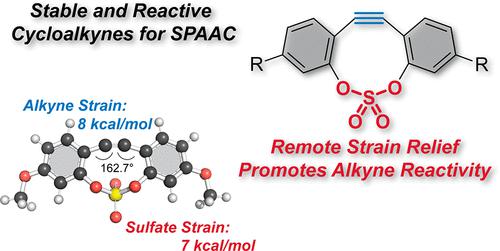
Remote Strain Activation in a Sulfate-Linked Dibenzocycloalkyne
Organic Letters ( IF 5.2 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.orglett.2c03397 Michael J Holzmann 1 , Namrata Khanal 1 , Pavel Yamanushkin 1 , Brian Gold 1
Organic Letters ( IF 5.2 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.orglett.2c03397 Michael J Holzmann 1 , Namrata Khanal 1 , Pavel Yamanushkin 1 , Brian Gold 1
Affiliation
|
Cycloalkynes and their utilization in cycloaddition reactions enable modular strategies spanning the molecular sciences. Strain─imparted by deviation from linearity─enables sufficient alkyne reactivity without the need for a catalyst (e.g., copper); however, the design and synthesis of stable reagents with suitable reactivity remains an ongoing challenge. We report the incorporation of an endocyclic sulfate within a dibenzocyclononyne scaffold to generate a cyclononyne displaying remarkable reactivity and stability. Through computational analyses, we revealed that the endocyclic sulfate group shares nearly half the total strain energy, providing an activation strategy that reduces alkyne bending. Rehybridization of alkyne carbons in the formation of the heterocyclic product relieves strain both at the reactive site and in the transannular sulfate group. This mode of remote activation enables rapid reactivity while minimizing distortion─and strain─at the reactive site (the alkyne). The result: a design strategy for a new class of cycloalkynes with increased stability and reactivity.
中文翻译:

硫酸盐连接的二苯并环炔烃中的远程应变激活
环炔及其在环加成反应中的应用使跨越分子科学的模块化策略成为可能。应变——因偏离线性而产生——无需催化剂(例如铜)即可实现足够的炔烃反应性;然而,设计和合成具有合适反应性的稳定试剂仍然是一个持续的挑战。我们报告了在二苯并环壬酮支架内掺入内环硫酸盐以生成显示出显着反应性和稳定性的环壬炔。通过计算分析,我们发现内环硫酸盐基团共享近一半的总应变能,提供了一种减少的激活策略炔烃弯曲。炔碳在杂环产物形成过程中的再杂化减轻了反应位点和环状硫酸基团中的应变。这种远程激活模式可实现快速反应,同时最大限度地减少反应部位(炔烃)的变形和应变。结果:一种新型环炔烃的设计策略具有更高的稳定性和反应性。
更新日期:2022-12-01
中文翻译:

硫酸盐连接的二苯并环炔烃中的远程应变激活
环炔及其在环加成反应中的应用使跨越分子科学的模块化策略成为可能。应变——因偏离线性而产生——无需催化剂(例如铜)即可实现足够的炔烃反应性;然而,设计和合成具有合适反应性的稳定试剂仍然是一个持续的挑战。我们报告了在二苯并环壬酮支架内掺入内环硫酸盐以生成显示出显着反应性和稳定性的环壬炔。通过计算分析,我们发现内环硫酸盐基团共享近一半的总应变能,提供了一种减少的激活策略炔烃弯曲。炔碳在杂环产物形成过程中的再杂化减轻了反应位点和环状硫酸基团中的应变。这种远程激活模式可实现快速反应,同时最大限度地减少反应部位(炔烃)的变形和应变。结果:一种新型环炔烃的设计策略具有更高的稳定性和反应性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号