当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bisphosphine/Nickel-Catalyzed C–O Cross-Coupling of Phenols with Chloropyridine and Related Electrophiles
Organic Letters ( IF 4.9 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.orglett.2c03587 Nicholas E Bodé 1 , Ryan T McGuire 1 , Mark Stradiotto 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.orglett.2c03587 Nicholas E Bodé 1 , Ryan T McGuire 1 , Mark Stradiotto 1
Affiliation
|
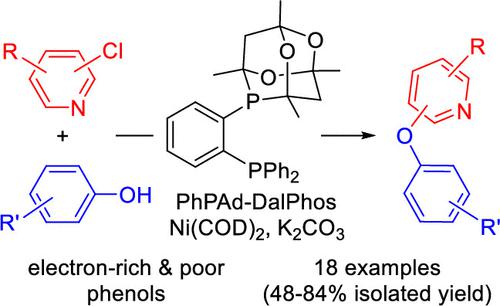
Notwithstanding recent developments in nickel-catalyzed C–O cross-coupling chemistry, such transformations of substituted phenols and (hetero)aryl chlorides with a useful reaction scope have yet to be reported. In this work, we disclose the results of catalyst screening that allowed the identification of PhPAd-DalPhos/NiCOD2 as an effective catalyst system under thermal conditions for the O-arylation of substituted phenols with chloropyridine-type electrophiles, leading to pyridyl-O-aryl frameworks that are found in active pharmaceutical ingredients.
中文翻译:

双膦/镍催化的苯酚与氯吡啶和相关亲电子试剂的 C-O 交叉偶联
尽管最近在镍催化的 C-O 交叉偶联化学方面取得了进展,但尚未报道具有有用反应范围的取代酚和(杂)芳基氯的这种转化。在这项工作中,我们公开了催化剂筛选的结果,该结果允许在热条件下将PhPAd-DalPhos/NiCOD 2鉴定为一种有效的催化剂体系,用于将取代的酚类与氯吡啶类亲电试剂进行 O-芳基化,从而生成吡啶基-O-在活性药物成分中发现的芳基骨架。
更新日期:2022-12-01
中文翻译:

双膦/镍催化的苯酚与氯吡啶和相关亲电子试剂的 C-O 交叉偶联
尽管最近在镍催化的 C-O 交叉偶联化学方面取得了进展,但尚未报道具有有用反应范围的取代酚和(杂)芳基氯的这种转化。在这项工作中,我们公开了催化剂筛选的结果,该结果允许在热条件下将PhPAd-DalPhos/NiCOD 2鉴定为一种有效的催化剂体系,用于将取代的酚类与氯吡啶类亲电试剂进行 O-芳基化,从而生成吡啶基-O-在活性药物成分中发现的芳基骨架。










































 京公网安备 11010802027423号
京公网安备 11010802027423号