当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Blue Light-Driven [4+2]-Cycloaddition: Diastereoselective Synthesis of Chromeno[4,3-b]quinoline and Chromeno[4,3-b][1,8]naphthyridine Scaffolds
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.joc.2c02380 Anil Ravi 1 , Gourishetty Srikanth 2 , Monther A Khanfar 3 , Raed A Al-Qawasmeh 4 , Mohammed I El-Gamal 1 , Taleb H Al-Tel
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-12-01 , DOI: 10.1021/acs.joc.2c02380 Anil Ravi 1 , Gourishetty Srikanth 2 , Monther A Khanfar 3 , Raed A Al-Qawasmeh 4 , Mohammed I El-Gamal 1 , Taleb H Al-Tel
Affiliation
|
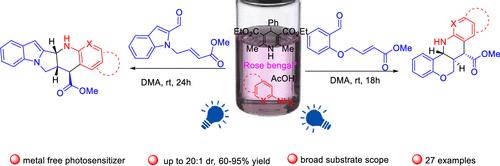
A one-pot, metal-free, light-driven [4+2]-cycloaddition reaction is described by accessing a diverse collection of chromeno[4,3-b]quinoline and chromeno[4,3-b][1,8]naphthyridine scaffolds in a diastereoselective manner. This process delivered stereoisomers, which were challenging to produce by an inverse-demand Diels–Alder reaction. The tetracyclic products were provided in good yields, promoted by rose bengal and blue light in a single operation. The developed protocol proceeded efficiently without the need for expensive photosensitizers such as Ir or Ru complexes. The cascade is modular and step-economic, and the substrate scope is wide. Polycyclic architectures can be assembled from readily available aniline, aminoazine, indole, and salicylaldehyde derivatives.
中文翻译:

蓝光驱动 [4+2]-环加成:铬并[4,3-b]喹啉和铬并[4,3-b][1,8]萘啶支架的非对映选择性合成
通过访问 chromeno[4,3- b ] 喹啉和 chromeno[4,3- b ][1,8 ]萘啶支架以非对映选择性方式。这个过程产生了立体异构体,而这些立体异构体很难通过逆需 Diels-Alder 反应来生产。四环产品以良好的收率提供,在一次操作中由玫瑰红和蓝光促进。开发的协议无需昂贵的光敏剂(如 Ir 或 Ru 复合物)即可高效进行。级联模块化,步进经济,底物范围广。多环结构可以由现成的苯胺、氨基嗪、吲哚和水杨醛衍生物组装而成。
更新日期:2022-12-01
中文翻译:

蓝光驱动 [4+2]-环加成:铬并[4,3-b]喹啉和铬并[4,3-b][1,8]萘啶支架的非对映选择性合成
通过访问 chromeno[4,3- b ] 喹啉和 chromeno[4,3- b ][1,8 ]萘啶支架以非对映选择性方式。这个过程产生了立体异构体,而这些立体异构体很难通过逆需 Diels-Alder 反应来生产。四环产品以良好的收率提供,在一次操作中由玫瑰红和蓝光促进。开发的协议无需昂贵的光敏剂(如 Ir 或 Ru 复合物)即可高效进行。级联模块化,步进经济,底物范围广。多环结构可以由现成的苯胺、氨基嗪、吲哚和水杨醛衍生物组装而成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号