当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly efficient and scalable total syntheses and stereochemical assignment of potent anti-inflammatory pesimquinolones I and J
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-12-01 , DOI: 10.1039/d2qo01788a Feng-Wei Guo 1, 2 , Tian-Yi Zhou 1 , Yong Qu 1, 2 , Mei-Yan Wei 1 , Guang-Ying Chen 3 , Yu-Cheng Gu 4 , Chang-Yun Wang 1, 2 , Chang-Lun Shao 1, 2, 3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-12-01 , DOI: 10.1039/d2qo01788a Feng-Wei Guo 1, 2 , Tian-Yi Zhou 1 , Yong Qu 1, 2 , Mei-Yan Wei 1 , Guang-Ying Chen 3 , Yu-Cheng Gu 4 , Chang-Yun Wang 1, 2 , Chang-Lun Shao 1, 2, 3
Affiliation
|
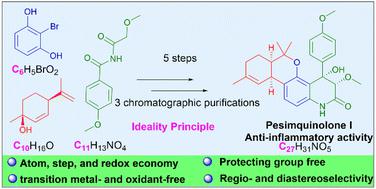
Based on the goals of sustainability and environmentally conscious science, great progress has been made in the field of green chemical synthesis, but a synthesis that combines high levels of step economy with high levels of efficiency and scalability has remained elusive. In this research, highly efficient and scalable total synthesis of potent anti-inflammatory pesimquinolone I (1) with a unique 6/6/6/6 tetracyclic core was accomplished starting from 2-bromoresorcinol and (1S,4R)-menthadienol in 5 steps. The approach adheres to the ideality principle, and the concepts of atom, step and redox economy, green chemistry, selectivity, and protecting group-free syntheses have been introduced. The synthetic strategy relies on a Lewis acid catalyzed Friedel–Crafts alkylation to form the tricyclic intermediate, a regioselective insertion of arynes into unsymmetric imides and a diastereoselective aldol cyclization to construct the tetracyclic skeleton. Syntheses and stereochemical assignment of the stereoisomer of pesimquinolone I and its analogues (15–19) were also accomplished. Compound 18 showed potent NO inhibitory effects with an IC50 value of 0.44 μM, which was stronger than that of the positive control (indomethacin, IC50 = 50.00 μM), and thus represents a potentially promising lead for anti-inflammatory drug discovery.
中文翻译:

高效和可扩展的全合成和有效抗炎 pesimquinolones I 和 J 的立体化学分配
基于可持续发展和具有环保意识的科学目标,绿色化学合成领域取得了巨大进展,但将高水平的步骤经济性与高水平的效率和可扩展性结合起来的合成仍然难以实现。在这项研究中,以2-溴间苯二酚和 (1 S , 4 R)-menthadienol 分 5 个步骤。该方法坚持理想性原则,引入了原子、步骤和氧化还原经济、绿色化学、选择性和无保护基合成等概念。合成策略依赖于路易斯酸催化的 Friedel-Crafts 烷基化形成三环中间体、芳烃区域选择性插入不对称酰亚胺和非对映选择性醛醇环化以构建四环骨架。pesimquinolone I 及其类似物 ( 15-19 ) 的立体异构体的合成和立体化学分配也已完成。化合物18显示出有效的 NO 抑制作用,IC 50值为 0.44 μM,强于阳性对照(吲哚美辛,IC 50= 50.00 μM),因此代表了抗炎药物发现的潜在有前途的线索。
更新日期:2022-12-05
中文翻译:

高效和可扩展的全合成和有效抗炎 pesimquinolones I 和 J 的立体化学分配
基于可持续发展和具有环保意识的科学目标,绿色化学合成领域取得了巨大进展,但将高水平的步骤经济性与高水平的效率和可扩展性结合起来的合成仍然难以实现。在这项研究中,以2-溴间苯二酚和 (1 S , 4 R)-menthadienol 分 5 个步骤。该方法坚持理想性原则,引入了原子、步骤和氧化还原经济、绿色化学、选择性和无保护基合成等概念。合成策略依赖于路易斯酸催化的 Friedel-Crafts 烷基化形成三环中间体、芳烃区域选择性插入不对称酰亚胺和非对映选择性醛醇环化以构建四环骨架。pesimquinolone I 及其类似物 ( 15-19 ) 的立体异构体的合成和立体化学分配也已完成。化合物18显示出有效的 NO 抑制作用,IC 50值为 0.44 μM,强于阳性对照(吲哚美辛,IC 50= 50.00 μM),因此代表了抗炎药物发现的潜在有前途的线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号