当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An unusual trans-hydrosilylation of prochiral 1,1-disubstituted cyclopropenes revealing the different nature of asymmetric palladium and rhodium catalysis
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-12-02 , DOI: 10.1039/d2qo01688e Han-Qi Zhou 1 , Fang-Ying Ling 1 , Xiao-Jun Fang 1 , Hua-Jie Zhu 1 , Li Li 1 , Fei Ye 1 , Zheng Xu 1 , Li-Wen Xu 1, 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-12-02 , DOI: 10.1039/d2qo01688e Han-Qi Zhou 1 , Fang-Ying Ling 1 , Xiao-Jun Fang 1 , Hua-Jie Zhu 1 , Li Li 1 , Fei Ye 1 , Zheng Xu 1 , Li-Wen Xu 1, 2
Affiliation
|
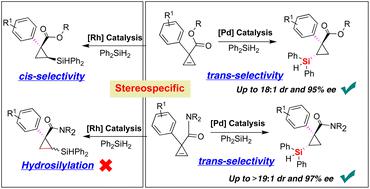
Catalytic asymmetric hydrosilylation is an extremely important and atom-economical chemical transformation in the field of catalysis and synthetic chemistry. Although stereospecific hydrosilylation provides a general strategy for elaborating diastereo- and enantioselective synthesis of optically pure organosilicon compounds, existing methods for accessing Si–C bond-forming hydrosilylation of alkenes rely almost entirely on terminal olefins. Herein, we reported a highly enantioselective palladium-catalyzed hydrosilylation reaction of 1,1-disubstituted carbonyl cyclopropenes with dihydrophenylsilane. We demonstrated that the palladium catalyst system provided stereodivergence to enable the trans-type diastereoselective synthesis of a wide variety of silylcyclopropanes bearing a quaternary carbon-stereocenter with good diastereo- and enantioselectivities (up to >19 : 1 dr and 97% ee). The preliminary experimental results of mechanistic studies showed that the steric repulsion between the TADDOL-derived phosphoramidite ligand and substrate would be an important factor, allowing access to different and reversed diastereospecific hydrosilylation in comparison to rhodium catalysis.
中文翻译:

前手性 1,1-二取代环丙烯的不寻常的反式氢化硅烷化揭示了不对称钯和铑催化的不同性质
催化不对称氢化硅烷化是催化和合成化学领域中极其重要的原子经济化学转化。尽管立体特异性氢化硅烷化为光学纯有机硅化合物的非对映选择性和对映选择性合成提供了一种通用策略,但现有的获得烯烃 Si-C 键形成氢化硅烷化的方法几乎完全依赖于末端烯烃。在此,我们报道了 1,1-二取代羰基环丙烯与二氢苯基硅烷的高度对映选择性钯催化氢化硅烷化反应。我们证明钯催化剂系统提供立体发散以实现反式具有季碳立体中心的多种甲硅烷基环丙烷的非对映选择性合成,具有良好的非对映和对映选择性(高达 >19 : 1 dr 和 97% ee)。机理研究的初步实验结果表明,TADDOL 衍生的亚磷酰胺配体与底物之间的空间排斥将是一个重要因素,与铑催化相比,允许获得不同的和反向的非对映特异性氢化硅烷化。
更新日期:2022-12-02
中文翻译:

前手性 1,1-二取代环丙烯的不寻常的反式氢化硅烷化揭示了不对称钯和铑催化的不同性质
催化不对称氢化硅烷化是催化和合成化学领域中极其重要的原子经济化学转化。尽管立体特异性氢化硅烷化为光学纯有机硅化合物的非对映选择性和对映选择性合成提供了一种通用策略,但现有的获得烯烃 Si-C 键形成氢化硅烷化的方法几乎完全依赖于末端烯烃。在此,我们报道了 1,1-二取代羰基环丙烯与二氢苯基硅烷的高度对映选择性钯催化氢化硅烷化反应。我们证明钯催化剂系统提供立体发散以实现反式具有季碳立体中心的多种甲硅烷基环丙烷的非对映选择性合成,具有良好的非对映和对映选择性(高达 >19 : 1 dr 和 97% ee)。机理研究的初步实验结果表明,TADDOL 衍生的亚磷酰胺配体与底物之间的空间排斥将是一个重要因素,与铑催化相比,允许获得不同的和反向的非对映特异性氢化硅烷化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号