当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ generated aminodiborane as a reagent for deoxygenative reduction of carboxamides to amines
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-12-01 , DOI: 10.1039/d2qo01717b Abhishek Nair 1 , Vikas Tiwari 1 , Ashutosh Verma 1 , Parul Saini 1 , Anil J. Elias 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-12-01 , DOI: 10.1039/d2qo01717b Abhishek Nair 1 , Vikas Tiwari 1 , Ashutosh Verma 1 , Parul Saini 1 , Anil J. Elias 1
Affiliation
|
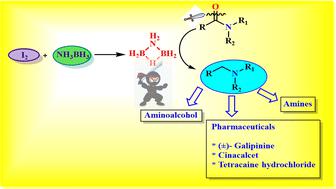
Herein, we report a new method for the synthesis of aminodiborane (μ-NH2B2H5) by the reaction of NH3BH3 and elemental iodine (I2). The in situ generated aminodiborane is used as a reagent for the reduction of carboxamides to amines. This method is applicable to obtain various secondary amides, tertiary amides, and trifluoroamides, with yields in the range of 67–94%. This protocol is also useful for preparing galipinine, cinacalcet and tetracaine hydrochloride, which are pharmaceutically important compounds. Control experiments and DFT studies have been carried out to explore the mechanistic pathway. These studies indicate that the active reagent in the reduction of secondary amides is aminodiborane and in the case of tertiary amides, aminodiborane and polyaminoborane.
中文翻译:

原位生成的氨基乙硼烷作为甲酰胺脱氧还原成胺的试剂
在此,我们报道了一种通过NH 3 BH 3和元素碘(I 2 )反应合成氨基乙硼烷(μ-NH 2 B 2 H 5 )的新方法。就地_生成的氨基二硼烷用作将甲酰胺还原成胺的试剂。该方法适用于制备各种仲酰胺、叔酰胺和三氟酰胺,收率在67%~94%之间。该方案也可用于制备加利品宁、西那卡塞和盐酸丁卡因,它们是药学上重要的化合物。已经进行了控制实验和 DFT 研究以探索机械途径。这些研究表明,还原仲酰胺的活性试剂是氨基二硼烷,而在叔酰胺的情况下,则是氨基二硼烷和聚氨基硼烷。
更新日期:2022-12-01
中文翻译:

原位生成的氨基乙硼烷作为甲酰胺脱氧还原成胺的试剂
在此,我们报道了一种通过NH 3 BH 3和元素碘(I 2 )反应合成氨基乙硼烷(μ-NH 2 B 2 H 5 )的新方法。就地_生成的氨基二硼烷用作将甲酰胺还原成胺的试剂。该方法适用于制备各种仲酰胺、叔酰胺和三氟酰胺,收率在67%~94%之间。该方案也可用于制备加利品宁、西那卡塞和盐酸丁卡因,它们是药学上重要的化合物。已经进行了控制实验和 DFT 研究以探索机械途径。这些研究表明,还原仲酰胺的活性试剂是氨基二硼烷,而在叔酰胺的情况下,则是氨基二硼烷和聚氨基硼烷。











































 京公网安备 11010802027423号
京公网安备 11010802027423号